Phase I Randomized Study of a Tetravalent Dengue Purified Inactivated Vaccine in Healthy Adults from Puerto Rico
- PMID: 29512481
- PMCID: PMC5953365
- DOI: 10.4269/ajtmh.17-0627
Phase I Randomized Study of a Tetravalent Dengue Purified Inactivated Vaccine in Healthy Adults from Puerto Rico
Abstract
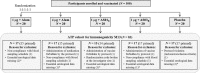
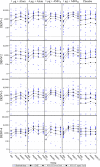
The safety and immunogenicity of four adjuvanted formulations of an investigational tetravalent dengue purified inactivated vaccine (DPIV) were evaluated in a predominantly dengue-primed population in Puerto Rico. In this placebo-controlled, randomized, observer-blind, phase I trial, 100 healthy adults were randomized 1:1:1:1:1 to receive DPIV at Day (D)0 and D28 (1 μg per dengue virus [DENV] type 1-4 adjuvanted with either alum, AS01E or AS03B, or 4 μg per DENV type adjuvanted with alum) or saline placebo. Functional antibody responses were assessed using a microneutralization assay at D56, Month (M)7, and M13. All DPIV formulations were well tolerated and no safety signals were identified through M13. The M13 according-to-protocol (ATP) immunogenicity cohort included 83 participants. The ATP analysis of immunogenicity was performed only on the 78 subjects seropositive for ≥ 1 DENV type at baseline: 69 tetravalent, three trivalent, two bivalent, and four monovalent. In all DPIV groups, geometric mean antibody titers (GMTs) increased from D0 to D56 and waned modestly through M13, while remaining well above prevaccination levels. The 4 μg + alum and the AS01E- and AS03B-adjuvanted formulations were highly immunogenic, with M13-neutralizing antibody GMTs against all four DENV types above 1,000. M13/D0 GMT ratios were highest in the 1 μg + AS03B group (ranging 3.2-3.7 depending on the DENV type). These results encourage continued clinical development of DPIV (ClinicalTrials.gov: NCT01702857).
Figures


References
-
- WHO , 2009. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control, New edition. Geneva, Switzerland: WHO Press.
-
- CDC , 2015. Dengue in Puerto Rico Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/dengue/about/inpuerto.html. Accessed April 2, 2017.
-
- Rigau-Perez JG, Vorndam AV, Clark GG, 2001. The dengue and dengue hemorrhagic fever epidemic in Puerto Rico, 1994–1995. Am J Trop Med Hyg 64: 67–74. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

