Evolved Cas9 variants with broad PAM compatibility and high DNA specificity
- PMID: 29512652
- PMCID: PMC5951633
- DOI: 10.1038/nature26155
Evolved Cas9 variants with broad PAM compatibility and high DNA specificity
Abstract
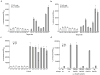
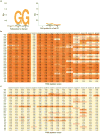
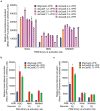
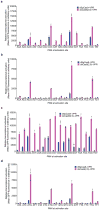
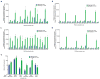
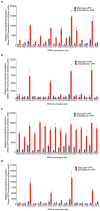
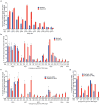
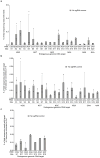
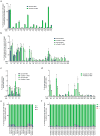
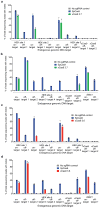
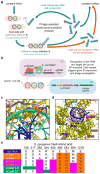
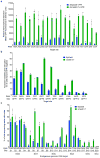
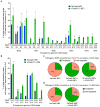
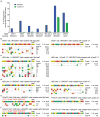
A key limitation of the use of the CRISPR-Cas9 system for genome editing and other applications is the requirement that a protospacer adjacent motif (PAM) be present at the target site. For the most commonly used Cas9 from Streptococcus pyogenes (SpCas9), the required PAM sequence is NGG. No natural or engineered Cas9 variants that have been shown to function efficiently in mammalian cells offer a PAM less restrictive than NGG. Here we use phage-assisted continuous evolution to evolve an expanded PAM SpCas9 variant (xCas9) that can recognize a broad range of PAM sequences including NG, GAA and GAT. The PAM compatibility of xCas9 is the broadest reported, to our knowledge, among Cas9 proteins that are active in mammalian cells, and supports applications in human cells including targeted transcriptional activation, nuclease-mediated gene disruption, and cytidine and adenine base editing. Notably, despite its broadened PAM compatibility, xCas9 has much greater DNA specificity than SpCas9, with substantially lower genome-wide off-target activity at all NGG target sites tested, as well as minimal off-target activity when targeting genomic sites with non-NGG PAMs. These findings expand the DNA targeting scope of CRISPR systems and establish that there is no necessary trade-off between Cas9 editing efficiency, PAM compatibility and DNA specificity.
Conflict of interest statement
The authors declare competing financial interests: J.H.H. and D.R.L. have filed patent applications on this work. D.R.L. is a consultant and co-founder of Editas Medicine, Beam Therapeutics, and Pairwise Plants, companies that use genome editing technologies. The authors declare no competing non-financial interests.
Figures

Comment in
-
Genetic engineering: Expanding the reach of Cas9.Nat Rev Genet. 2018 May;19(5):250-251. doi: 10.1038/nrg.2018.15. Epub 2018 Mar 12. Nat Rev Genet. 2018. PMID: 29527014 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

