MicroRNA-155 inhibits the osteogenic differentiation of mesenchymal stem cells induced by BMP9 via downregulation of BMP signaling pathway
- PMID: 29512689
- PMCID: PMC5881775
- DOI: 10.3892/ijmm.2018.3526
MicroRNA-155 inhibits the osteogenic differentiation of mesenchymal stem cells induced by BMP9 via downregulation of BMP signaling pathway
Abstract
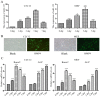
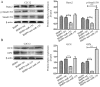
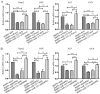
Previous studies have indicated that bone morphogenetic protein 9 (BMP9) can promote the osteogenic differentiation of mesenchymal stem cells (MSCs) and increase bone formation in bone diseases. However, the mechanisms involved remained poorly understood. It is necessary to investigate the specific regulatory mechanisms of osteogenic differentiation that were induced by BMP9. During the process of osteogenic differentiation induced by BMP9, the expression of microRNA-155 (miR-155) exhibited a tendency of increasing at first and then decreasing, which made us consider that miR-155 may have a modulatory role in this process, but the roles of this process have not been elucidated. This study aimed to uncover miR-155 capable of concomitant regulation of this process. mmu-miR-155 mimic (miR-155) was transfected into MSCs and osteogenesis was induction by using recombinant adenovirus expressing BMP9. Overexpressed miR-155 in MSCs led to a decrease in alkaline phosphatase (ALP) staining and Alizarin red S staining during osteogenic differentiation, and reduced the expression of osteogenesis-related genes, such as runt-related transcription factor 2 (Runx2), osterix (OSX), osteocalcin (OCN) and osteopontin (OPN). On protein levels, overexpressed miR-155 markedly decreased the expression of phosphorylated Smad1/5/8 (p-Smad1/5/8), Runx2, OCN and OPN. Luciferase reporter assay revealed Runx2 and bone morphogenetic protein receptor 9 (BMPR2) are two direct target genes of miR-155. Downregulation of the expression of Runx2 and BMPR2, respectively could offset the inhibitory effect of miR-155 in the osteogenesis of MSCs. In vivo, subcutaneous ectopic osteogenesis of MSCs in nude mice showed miR-155 inhibited osteogenic differentiation. In conclusion, our results demonstrated that miR-155 can inhibit the osteogenic differentiation induced by BMP9 in MSCs.
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

