PEDF protects cardiomyocytes by promoting FUNDC1‑mediated mitophagy via PEDF-R under hypoxic condition
- PMID: 29512692
- PMCID: PMC5881750
- DOI: 10.3892/ijmm.2018.3536
PEDF protects cardiomyocytes by promoting FUNDC1‑mediated mitophagy via PEDF-R under hypoxic condition
Abstract
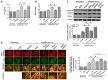
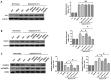
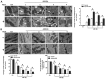
Pigment epithelial-derived factor (PEDF) is known to exert diverse physiological activities. Previous studies suggest that hypoxia could induce mitophagy. Astoundingly, under hypoxic condition, we found that PEDF decreased the mitochondrial density of cardiomyocytes. In this study, we evaluated whether PEDF could decrease the mitochondrial density and play a protective role in hypoxic cardiomyocytes via promoting mitophagy. Immunostaining and western blotting were used to analyze mitochondrial density and mitophagy of hypoxic cardiomyocytes. Gas chromatography‑mass spectrometry and ELISA were used to analyze levels of palmitic acid and diacylglycerol. Transmission Electron Microscopy was used to detect mitophagy and the mitochondrial density in adult male Sprague-Dawley rat model of acute myocardial infarction. Compared to the control group, we observed that PEDF decreased mitochondrial density through promoting hypoxic cardiomyocyte mitophagy. PEDF increased the levels of palmitic acid and diacylglycerol, and then upregulated the levels of protein kinase Cα (PKC-α) and its activation. Furthermore, inhibition of PKC-α by Go6976 could effectively suppress PEDF-induced mitophagy. Besides, we found that PEDF promoted FUNDC1-mediated cardiomyocyte mitophagy via ULK1, which depended on the activation of PKC-α. Finally, we discovered that mitophagy was increased and mitochondrial density was reduced in adult male Sprague-Dawley rat model of acute myocardial infarction. We concluded that PEDF promotes mitophagy to protect hypoxic cardiomyocytes, through PEDF/PEDF-R/PA/DAG/PKC-α/ULK1/FUNDC1 pathway.
Figures




Similar articles
-
PEDF Inhibits the Activation of NLRP3 Inflammasome in Hypoxia Cardiomyocytes through PEDF Receptor/Phospholipase A2.Int J Mol Sci. 2016 Dec 12;17(12):2064. doi: 10.3390/ijms17122064. Int J Mol Sci. 2016. PMID: 27973457 Free PMC article.
-
A decrease of ATP production steered by PEDF in cardiomyocytes with oxygen-glucose deprivation is associated with an AMPK-dependent degradation pathway.Int J Cardiol. 2018 Apr 15;257:262-271. doi: 10.1016/j.ijcard.2018.01.034. Epub 2018 Jan 17. Int J Cardiol. 2018. PMID: 29361350
-
Pigment epithelium-derived factor promotes Fas-induced cardiomyocyte apoptosis via its receptor phospholipase A2.Life Sci. 2014 Mar 18;99(1-2):18-23. doi: 10.1016/j.lfs.2013.07.013. Epub 2013 Jul 24. Life Sci. 2014. PMID: 23892196
-
Roles of pigment epithelium-derived factor in cardiomyocytes: implications for use as a cardioprotective therapeutic.J Pharm Pharmacol. 2023 Jun 5;75(6):746-757. doi: 10.1093/jpp/rgad037. J Pharm Pharmacol. 2023. PMID: 37104852 Review.
-
[Systematic analysis of magnesium dependent mitochondrial proteins].Kardiologiia. 2014;54(9):86-92. doi: 10.18565/cardio.2014.9.86-92. Kardiologiia. 2014. PMID: 25702408 Review. Russian.
Cited by
-
Mitophagy in Cardiovascular Diseases.J Clin Med. 2020 Mar 24;9(3):892. doi: 10.3390/jcm9030892. J Clin Med. 2020. PMID: 32214047 Free PMC article. Review.
-
Mitophagy in Hepatic Insulin Resistance: Therapeutic Potential and Concerns.Front Pharmacol. 2019 Oct 10;10:1193. doi: 10.3389/fphar.2019.01193. eCollection 2019. Front Pharmacol. 2019. PMID: 31649547 Free PMC article. Review.
-
Advances in the mechanism and treatment of mitochondrial quality control involved in myocardial infarction.J Cell Mol Med. 2021 Aug;25(15):7110-7121. doi: 10.1111/jcmm.16744. Epub 2021 Jun 23. J Cell Mol Med. 2021. PMID: 34160885 Free PMC article. Review.
-
Exploiting Mitochondria by Triggering a Faulty Unfolded Protein Response Leads to Effective Cardioprotection.Int J Med Sci. 2025 Jan 1;22(1):188-196. doi: 10.7150/ijms.100523. eCollection 2025. Int J Med Sci. 2025. PMID: 39744160 Free PMC article.
-
MitoTEMPOL modulates mitophagy and histopathology of Wistar rat liver after streptozotocin injection.Iran J Basic Med Sci. 2022 Nov;25(11):1382-1388. doi: 10.22038/IJBMS.2022.65285.14375. Iran J Basic Med Sci. 2022. PMID: 36474569 Free PMC article.
References
-
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics - 2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. - DOI - PubMed
-
- Begieneman MP, van de Goot FR, Fritz J, Rozendaal R, Krijnen PA, Niessen HW. Validation of ultrastructural analysis of mitochondrial deposits in cardiomyocytes as a method of detecting early acute myocardial infarction in humans. J Forensic Sci. 2010;55:988–992. doi: 10.1111/j.1556-4029.2010.01377.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

