The Biology of Regeneration Failure and Success After Spinal Cord Injury
- PMID: 29513146
- PMCID: PMC5966716
- DOI: 10.1152/physrev.00017.2017
The Biology of Regeneration Failure and Success After Spinal Cord Injury
Abstract
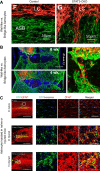
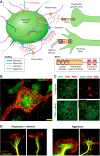
Since no approved therapies to restore mobility and sensation following spinal cord injury (SCI) currently exist, a better understanding of the cellular and molecular mechanisms following SCI that compromise regeneration or neuroplasticity is needed to develop new strategies to promote axonal regrowth and restore function. Physical trauma to the spinal cord results in vascular disruption that, in turn, causes blood-spinal cord barrier rupture leading to hemorrhage and ischemia, followed by rampant local cell death. As subsequent edema and inflammation occur, neuronal and glial necrosis and apoptosis spread well beyond the initial site of impact, ultimately resolving into a cavity surrounded by glial/fibrotic scarring. The glial scar, which stabilizes the spread of secondary injury, also acts as a chronic, physical, and chemo-entrapping barrier that prevents axonal regeneration. Understanding the formative events in glial scarring helps guide strategies towards the development of potential therapies to enhance axon regeneration and functional recovery at both acute and chronic stages following SCI. This review will also discuss the perineuronal net and how chondroitin sulfate proteoglycans (CSPGs) deposited in both the glial scar and net impede axonal outgrowth at the level of the growth cone. We will end the review with a summary of current CSPG-targeting strategies that help to foster axonal regeneration, neuroplasticity/sprouting, and functional recovery following SCI.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

