Cannabis-based medicines for chronic neuropathic pain in adults
- PMID: 29513392
- PMCID: PMC6494210
- DOI: 10.1002/14651858.CD012182.pub2
Cannabis-based medicines for chronic neuropathic pain in adults
Abstract
Background: This review is one of a series on drugs used to treat chronic neuropathic pain. Estimates of the population prevalence of chronic pain with neuropathic components range between 6% and 10%. Current pharmacological treatment options for neuropathic pain afford substantial benefit for only a few people, often with adverse effects that outweigh the benefits. There is a need to explore other treatment options, with different mechanisms of action for treatment of conditions with chronic neuropathic pain. Cannabis has been used for millennia to reduce pain. Herbal cannabis is currently strongly promoted by some patients and their advocates to treat any type of chronic pain.
Objectives: To assess the efficacy, tolerability, and safety of cannabis-based medicines (herbal, plant-derived, synthetic) compared to placebo or conventional drugs for conditions with chronic neuropathic pain in adults.
Search methods: In November 2017 we searched CENTRAL, MEDLINE, Embase, and two trials registries for published and ongoing trials, and examined the reference lists of reviewed articles.
Selection criteria: We selected randomised, double-blind controlled trials of medical cannabis, plant-derived and synthetic cannabis-based medicines against placebo or any other active treatment of conditions with chronic neuropathic pain in adults, with a treatment duration of at least two weeks and at least 10 participants per treatment arm.
Data collection and analysis: Three review authors independently extracted data of study characteristics and outcomes of efficacy, tolerability and safety, examined issues of study quality, and assessed risk of bias. We resolved discrepancies by discussion. For efficacy, we calculated the number needed to treat for an additional beneficial outcome (NNTB) for pain relief of 30% and 50% or greater, patient's global impression to be much or very much improved, dropout rates due to lack of efficacy, and the standardised mean differences for pain intensity, sleep problems, health-related quality of life (HRQoL), and psychological distress. For tolerability, we calculated number needed to treat for an additional harmful outcome (NNTH) for withdrawal due to adverse events and specific adverse events, nervous system disorders and psychiatric disorders. For safety, we calculated NNTH for serious adverse events. Meta-analysis was undertaken using a random-effects model. We assessed the quality of evidence using GRADE and created a 'Summary of findings' table.
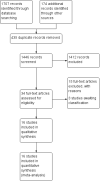
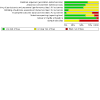
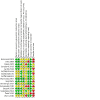
Main results: We included 16 studies with 1750 participants. The studies were 2 to 26 weeks long and compared an oromucosal spray with a plant-derived combination of tetrahydrocannabinol (THC) and cannabidiol (CBD) (10 studies), a synthetic cannabinoid mimicking THC (nabilone) (two studies), inhaled herbal cannabis (two studies) and plant-derived THC (dronabinol) (two studies) against placebo (15 studies) and an analgesic (dihydrocodeine) (one study). We used the Cochrane 'Risk of bias' tool to assess study quality. We defined studies with zero to two unclear or high risks of bias judgements to be high-quality studies, with three to five unclear or high risks of bias to be moderate-quality studies, and with six to eight unclear or high risks of bias to be low-quality studies. Study quality was low in two studies, moderate in 12 studies and high in two studies. Nine studies were at high risk of bias for study size. We rated the quality of the evidence according to GRADE as very low to moderate.Primary outcomesCannabis-based medicines may increase the number of people achieving 50% or greater pain relief compared with placebo (21% versus 17%; risk difference (RD) 0.05 (95% confidence interval (CI) 0.00 to 0.09); NNTB 20 (95% CI 11 to 100); 1001 participants, eight studies, low-quality evidence). We rated the evidence for improvement in Patient Global Impression of Change (PGIC) with cannabis to be of very low quality (26% versus 21%;RD 0.09 (95% CI 0.01 to 0.17); NNTB 11 (95% CI 6 to 100); 1092 participants, six studies). More participants withdrew from the studies due to adverse events with cannabis-based medicines (10% of participants) than with placebo (5% of participants) (RD 0.04 (95% CI 0.02 to 0.07); NNTH 25 (95% CI 16 to 50); 1848 participants, 13 studies, moderate-quality evidence). We did not have enough evidence to determine if cannabis-based medicines increase the frequency of serious adverse events compared with placebo (RD 0.01 (95% CI -0.01 to 0.03); 1876 participants, 13 studies, low-quality evidence).Secondary outcomesCannabis-based medicines probably increase the number of people achieving pain relief of 30% or greater compared with placebo (39% versus 33%; RD 0.09 (95% CI 0.03 to 0.15); NNTB 11 (95% CI 7 to 33); 1586 participants, 10 studies, moderate quality evidence). Cannabis-based medicines may increase nervous system adverse events compared with placebo (61% versus 29%; RD 0.38 (95% CI 0.18 to 0.58); NNTH 3 (95% CI 2 to 6); 1304 participants, nine studies, low-quality evidence). Psychiatric disorders occurred in 17% of participants using cannabis-based medicines and in 5% using placebo (RD 0.10 (95% CI 0.06 to 0.15); NNTH 10 (95% CI 7 to 16); 1314 participants, nine studies, low-quality evidence).We found no information about long-term risks in the studies analysed.Subgroup analysesWe are uncertain whether herbal cannabis reduces mean pain intensity (very low-quality evidence). Herbal cannabis and placebo did not differ in tolerability (very low-quality evidence).
Authors' conclusions: The potential benefits of cannabis-based medicine (herbal cannabis, plant-derived or synthetic THC, THC/CBD oromucosal spray) in chronic neuropathic pain might be outweighed by their potential harms. The quality of evidence for pain relief outcomes reflects the exclusion of participants with a history of substance abuse and other significant comorbidities from the studies, together with their small sample sizes.
Conflict of interest statement
MM: none known; MM is a specialist in palliative care who treats patients with chronic neuropathic pain.
TP: none known; TP is a specialist pain physician and manages patients with neuropathic pain.
LR: none known; PR is a specialist in palliative care who treats patients with chronic neuropathic pain.
FP is a specialist in pain medicine who treats patients with chronic neuropathic pain. He has received speaking fees for one educational lecture for Janssen‐Cilaq (2015) on fibromyalgia and participated in an advisory board for the same company focusing on an unrelated product (2015).
WH is a specialist in general internal medicine, psychosomatic medicine and pain medicine, who treats patients with fibromyalgia and chronic neuropathic pain. He is a member of the medical board of the German Fibromyalgia Association. He is the head of the steering committee of the German guideline on fibromyalgia and a member of the steering committee of the European League Against Rheumatism (EULAR) update recommendations on the management of fibromyalgia. He received speaking fees for one educational lecture from Grünenthal (2015) on pain management.
Figures











Update of
- doi: 10.1002/14651858.CD012182
References
References to studies included in this review
Bermann 2004 {published data only}
-
- Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain 2004;112:299-306. - PubMed
Ellis 2009 {published data only}
Frank 2008 {published data only}
Langford 2013 {published data only}
-
- Langford RM, Mares J, Novotna A, Vachova M, Novakova I, Notcutt W, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. Journal of Neurology 2013;260:984-97. - PubMed
Lynch 2014 {published data only}
-
- Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. Journal of Pain and Symptom Management 2014;47:166-73. - PubMed
NCT00710424 {published data only}
-
- NCT 00710424. A study of Sativex® for pain relief due to diabetic neuropathy. clinicaltrials.gov/ct2/results?term=NCT00710424+&Search=Search (first Posted 4 July 2008).
NCT01606176 {published data only}
-
- NCT01606176. A Study to Evaluate the Effects of Cannabis Based Medicine in Patients With Pain of Neurological Origin. clinicaltrials.gov/ct2/results?cond=&term=NCT01606176&cntry1=&am... (first posted 25 May 2012).
NCT01606202 {published data only}
-
- NCT 01606202. A study of cannabis based medicine extracts and placebo in patients with pain due to spinal cord injury. clinicaltrials.gov/ct2/results?term= NCT 01606202&Search=Search (first posted 25 May 2012).
Nurmikko 2007 {published data only}
-
- Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain 2007;133:210-20. - PubMed
Rog 2005 {published data only}
-
- Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005;65:812-9. - PubMed
Schimrigk 2017 {published data only}
Selvarajah 2010 {published data only}
Serpell 2014 {published data only}
-
- Serpell M, Ratcliffe S, Hovorka J, Schofield M, Taylor L, Lauder H, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. European Journal of Pain 2014;18:999-1012. - PubMed
Svendsen 2004 {published data only}
Toth 2012 {published data only}
-
- Toth C, Mawani S, Brady S, Chan C, Liu C, Mehina E, et al. An enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic pain. Pain 2012;153:2073-82. - PubMed
References to studies excluded from this review
Abrams 2007 {published data only}
-
- Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology 2007;68:515-21. - PubMed
Corey‐Bloom 2012 {published data only}
Karst 2003 {published data only}
-
- Karst M, Salim K, Burstein S, Conrad I, Hoy L, Schneider U. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial. JAMA 2003;290:1757-62. - PubMed
Notcutt 2011 {published data only}
-
- Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 'N of 1' studies. Anaesthesia 2004;59:440-52. - PubMed
Novotna 2011 {published data only}
-
- Novotna A, Mares J, Ratcliffe S, Novakova I, Vachova M, Zapletalova O, et al. Arandomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex(®)), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. European Journal of Neurology 2011;18:1122-31. - PubMed
Rintala 2010 {published data only}
-
- Rintala DH, Fiess RN, Tan G, Holmes SA, Bruel BM. Effect of dronabinol on central neuropathic pain after spinal cord injury: a pilot study. American Journal of Physical Medicine & Rehabilitation 2010;89:840-8. - PubMed
Turcotte 2015 {published data only}
-
- Turcotte D, Doupe M, Torabi M, Gomori A, Ethans K, Esfahani F, et al. Nabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: a randomized controlled trial. Pain Medicine 2015;16:149-59. - PubMed
Wade 2003 {published data only}
-
- Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clinical Rehabilitation 2003;17:21-9. - PubMed
Wade 2004 {published data only}
-
- Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Multiple Sclerosis 2004;10:434-41. - PubMed
Wallace 2015 {published data only}
Wilsey 2008 {published data only}
Wilsey 2013 {published data only}
Wissel 2006 {published data only}
-
- Wissel J, Haydn T, Müller J, Brenneis C, Berger T, Berger T. Low dose treatment with the synthetic cannabinoid Nabilone significantly reduces spasticity-related pain: a double-blind placebo-controlled cross-over trial. Journal of Neurology 2006;253:1337-41. - PubMed
Zajicek 2003 {published data only}
-
- Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn A, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomized placebo-controlled trial. Lancet 2003;362:1517-26. - PubMed
Zajicek 2012 {published data only}
-
- Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG, MUSEC Research Group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. Journal of Neurology, Neurosurgery and Psychiatry 2012;83:1125-32. - PubMed
References to studies awaiting assessment
NCT00699634 {published data only}
-
- NCT00699634. Nabilone for the treatment of phantom limb pain. clinicaltrials.gov/ct2/show/NCT00699634?term=NCT00699634&rank=1 (first posted 18 June 18).
NCT01035281 {published data only}
-
- NCT01035281. Efficacy study of nabilone in the treatment of diabetic peripheral neuropathic pain. clinicaltrials.gov/ct2/show/NCT01035281?term=NCT01035281&rank=1 (first posted 18 December 2009).
NCT01222468 {published data only}
-
- NCT01222468. Effect of cannabinoids on spasticity and neuropathic pain in spinal cord injured persons. clinicaltrials.gov/ct2/show/NCT01222468?term=NCT01222468&rank=1 (first posted 18 October 2010).
Additional references
Ablin 2016
-
- Ablin J, Ste-Marie PA, Schäfer M, Häuser W, Fitzcharles MA. Medical use of cannabis products: lessons to be learned from Israel and Canada. Schmerz 2016;30:3-13. - PubMed
AlBalawi 2013
Andersohn 2008
-
- Andersohn F, Garbe E. Pharmacoepidemiological research with large health databases. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2008;51:1134-55. - PubMed
Andreae 2015
Attal 2016
-
- Attal N, Andrade DC, Adam F, Ranoux D, Teixeira MJ, Galhardoni R, et al. Safety and efficacy of repeated injections of botulinum toxin A in peripheral neuropathic pain (BOTNEP): a randomised, double-blind, placebo-controlled trial. Lancet Neurology 2016;15:555-65. - PubMed
Baron 2004
-
- Baron R, Binder A. How neuropathic is sciatica? The mixed pain concept [Wie neuropathisch ist die lumboischialgie? Das gemischte schmerz konzept]. Orthopäde 2004;33:568-75. - PubMed
Baron 2012
Baron 2017
Berger 2004
Berger 2009
Berger 2012
Bouhassira 2008
-
- Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008;136:380-7. - PubMed
Boychuk 2015
-
- Boychuk DG, Goddard G, Mauro G, Orellana MF. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. Journal of Oral and Facial Pain and Headache 2015;29(1):7-14. - PubMed
Calvo 2012
Clauw 2015
-
- Clauw D. What is the meaning of "small fiber neuropathy" in fibromyalgia? Pain 2015;156(11):2115-6. - PubMed
Cochrane PaPaS 2012
-
- Cochrane Pain, Palliative and Supportive Care Group. PaPaS author and referee guidance. papas.cochrane.org/papas-documents (accessed 5 June 2015).
Cohen 1988
-
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale: Lawrence Erlbaum Associates, 1988.
De Vries 2014
-
- De Vries M, Van Rijckevorsel DC, Wilder-Smith OH, Van Goor H. Dronabinol and chronic pain: importance of mechanistic considerations. Expert Opinion on Pharmacotherapy 2014;15:1525-34. - PubMed
Dechartes 2013
Dechartres 2014
Demant 2014
-
- Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain 2014;155(11):2263-73. [DOI: 10.1016/j.pain.2014.08.014] - DOI - PubMed
Derry 2012
Derry 2014
Derry 2017
Dworkin 2008
Dworkin 2013
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. - PubMed
European Medicines Agency 2007
-
- Guideline on clinical medicinal products intended for the treatment of neuropathic pain. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/... 2007 (Accessed May 2, 2015).
Fayers 2014
Finnerup 2013
Finnerup 2015
Fitzcharles 2014
-
- Fitzcharles MA, Clauw DJ, Ste-Marie PA, Shir Y. The dilemma of medical marijuana use by rheumatology patients. Arthritis Care and Research 2014;66:797-801. - PubMed
Furukawa 2005
-
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta-analyses. International Clinical Psychopharmacology 2005;20:49-52. - PubMed
Gaskell 2016
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guindon 2009
Gustorff 2008
-
- Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, et al. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiologica Scandinavica 2008;52:132-6. - PubMed
Guyatt 2013a
-
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66:151-7. - PubMed
Guyatt 2013b
-
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. Journal of Clinical Epidemiology 2013;66:158-72. - PubMed
Hall 2008
Helfert 2015
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hillard 2012
Hoggart 2015
-
- Hoggart B, Ratcliffe S, Ehler E, Simpson KH, Hovorka J, Lejčko J, et al. A multicentre, open-label, follow-on study to assess the long-term maintenance of effect, tolerance and safety of THC/CBD oromucosal spray in the management of neuropathic pain. Journal of Neurology 2015;262:27-40. - PubMed
Häuser 2012
-
- Häuser W, Bartram C, Bartram-Wunn E, Tölle T. Adverse events attributable to nocebo in randomized controlled drug trials in fibromyalgia syndrome and painful diabetic peripheral neuropathy: systematic review. Clinical Journal of Pain 2012;28:437-51. - PubMed
Häuser 2016
Häuser 2017
Häuser 2018
-
- Häuser W, Petzke F, Fitzcharles MA. Efficacy, tolerability and safety of cannabis-based medicines for chronic pain management - an overview of systematic reviews. European Journal of Pain 2018;22:455-70. - PubMed
International Council for Harmonisation 2016
-
- International Council for Harmonisation. Medical Dictionary for Regulatory Activities Version 19.1. www.meddra.org/news-and-events/news/all-translations-meddra-version-191-... 2016.
Jensen 2011
Jensen 2015
-
- Jensen B, Chen J, Furnish T, Wallace M. Medical marijuana and chronic pain: a review of basic science and clinical evidence. Current Pain Headache Reports 2015;19:524. - PubMed
Kalant 2001
-
- Kalant H. Medicinal use of cannabis: history and current status. Pain Research & Management 2001;6:80-91. - PubMed
Kalso 2013
-
- Kalso E, Aldington DJ, Moore RA. Drugs for neuropathic pain. BMJ 2013;347:f7339. - PubMed
Katusic 1991
-
- Katusic S, Williams DB, Beard CM, Bergstralh EJ, Kurland LT. Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota,1945-1984. Neuroepidemiology 1991;10:276-81. - PubMed
Koopman 2009
-
- Koopman JS, Dieleman JP, Huygen FJ, De Mos M, Martin CG, Sturkenboom MC. Incidence of facial pain in the general population. Pain 2009;147:122-7. - PubMed
Koppel 2014
Krcevski‐Skvarc 2018
-
- Krcevski-Skvarc N, Wells C, Häuser W. Availability and approval of cannabis- based medicines for chronic pain management and palliative/supportive care in Europe – a survey of the status in the chapters of the European Pain Federation. European Journal Pain 2018;22:440-54. - PubMed
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Annals of Internal Medicine 1987;107:224-33. - PubMed
Lee 2013
Long 2009
Lunn 2014
Lynch 2011
McQuay 1998
-
- McQuay H, Moore R. An Evidence-Based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 0-19-263048-2]
McQuay 2007
-
- McQuay HJ, Smith LA, Moore RA. Chronic pain. In: In: Stevens A, Raftery J, Mant J, Simpson S, editor(s). Health Care Needs Assessment, 3rd Series. Radcliffe Publishing, 2007.
Mintzes 2015
-
- Mintzes B, Lexchin J, Quintano AS. Clinical trial transparency: many gains but access to evidence for new medicines remains imperfect. British Medical Bulletin 2015;116:43-53. - PubMed
Moher 2009
Moore 1998
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA, editors(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15-24. [ISBN: 978-0-931092-69-5]
Moore 2009
Moore 2010a
Moore 2010b
Moore 2010c
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374-9. [DOI: 10.1136/ard.2009.107805] - DOI - PMC - PubMed
Moore 2010d
Moore 2011a
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta-analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982-9. - PubMed
Moore 2011b
Moore 2012
Moore 2013a
-
- Moore RA, Straube S, Aldington D. Pain measures and cut-offs - 'no worse than mild pain' as a simple, universal outcome. Anesthesia 2013;68:400-12. - PubMed
Moore 2013b
Moore 2014a
Moore 2014b
Moore 2015a
Moore 2015b
Moore 2017
Moulin 2014
NICE 2013
-
- National Institute for Health and Care Excellence (NICE). Neuropathic pain - pharmacological management: the pharmacological management of neuropathic pain in adults in non-specialist settings, 2013. www.nice.org.uk/guidance/cg173 (accessed 19 October 2014).
Nüesch 2010
Owens 2015
-
- Owens B. Drug development: the treasure chest. Nature 2015;525:S6-S8. - PubMed
Petzke 2016
-
- Petzke F, Enax-Krumova EK, Häuser W. Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: a systematic review of randomized controlled studies. Schmerz 2016;30:62-88. - PubMed
Rappaport 1994
-
- Rappaport ZH, Devor M. Trigeminal neuralgia: the role of self-sustaining discharge in the trigeminal ganglion. Pain 1994;56:127-38. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schaefert 2015
-
- Schaefert R, Welsch P, Klose P, Sommer C, Petzke F, Häuser W. Opioids in chronic osteoarthritis pain. A systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration [Opioide bei chronischem arthroseschmerz. Systematische übersicht und metaanalyse der wirksamkeit, verträglichkeit und sicherheit in randomisierten, placebokontrollierten studien über mindestens 4 wochen]. Schmerz 2015;29:47-59. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Scott 2006
Smith 2016
-
- Smith SM, Amtmann D, Askew RL, Gewandter JS, Hunsinger M, Jensen MP, et al. Pain intensity rating training: results from an exploratory study of the ACTTION PROTECCT system. Pain 2016;157(5):1056-64. - PubMed
Sommer 2015
-
- Sommer C, Welsch P, Klose P, Schaefert R, Petzke F, Häuser W. Opioids in chronic neuropathic pain. A systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration [Opioide bei chronischem neuropathischem schmerz. Systematische übersicht und metaanalyse der wirksamkeit, verträglichkeit und sicherheit in randomisierten, placebokontrollierten studien über mindestens 4 wochen]. Schmerz 2015;29:35-46. - PubMed
Stannard 2016
Straube 2008
-
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrolment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266-75. [DOI: 10.1111/j.1365-2125.2008.03200.] - DOI - PMC - PubMed
Straube 2010
Sultan 2008
Thorlund 2011
Thyson 2014
-
- Tyson SF, Brown P. How to measure pain in neurological conditions? A systematic review of psychometric properties and clinical utility of measurement tools. Clinical Rehabilitation 2014;28:669-86. - PubMed
Torrance 2006
-
- Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. Journal of Pain 2006;7:281-9. - PubMed
Treede 2008
Turner 2013
Van Hecke 2014
-
- Van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 2014;155:654-62. - PubMed
Van Hoek 2009
Volkow 2014
von Hehn 2012
Vos 2012
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163-96. [DOI: 10.1016/S0140-6736(12)61729-2] - DOI - PMC - PubMed
Ware 2015
-
- Ware MA, Wang T, Shapiro S, Collet JP, COMPASS study team. Cannabis for the management of pain: assessment of safety study (COMPASS). Journal of Pain 2015;16:1233-42. - PubMed
Whiting 2015
-
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 2015;313:2456-73. - PubMed
Wiffen 2013
Zettl 2016
Zhang 2015
-
- Zhang J, Echeverry S, Lim TK, Lee SH, Shi XQ, Huang H. Can modulating inflammatory response be a good strategy to treat neuropathic pain? Current Pharmaceutical Design 2015;21:831-9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

