The Molecular Industrial Revolution: Automated Synthesis of Small Molecules
- PMID: 29513400
- PMCID: PMC5912692
- DOI: 10.1002/anie.201710482
The Molecular Industrial Revolution: Automated Synthesis of Small Molecules
Abstract
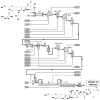
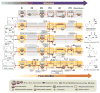
Today we are poised for a transition from the highly customized crafting of specific molecular targets by hand to the increasingly general and automated assembly of different types of molecules with the push of a button. Creating machines that are capable of making many different types of small molecules on demand, akin to that which has been achieved on the macroscale with 3D printers, is challenging. Yet important progress is being made toward this objective with two complementary approaches: 1) Automation of customized synthesis routes to different targets by machines that enable the use of many reactions and starting materials, and 2) automation of generalized platforms that make many different targets using common coupling chemistry and building blocks. Continued progress in these directions has the potential to shift the bottleneck in molecular innovation from synthesis to imagination, and thereby help drive a new industrial revolution on the molecular scale.
Keywords: artificial intelligence; flow chemistry; iterative synthesis; machine learning; small molecules.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures






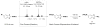











References
-
- Harmand S, Lewis JE, Feibel CS, Lepre CJ, Prat S, Lenoble A, Boës X, Quinn RL, Brenet M, Arroyo A, et al. Nature. 2015;521:310. - PubMed
-
- Bautista Paz E, Ceccarelli M, Echávarri Otero J, Muñoz Sanz JL. A Brief Illustrated History of Machines and Mechanisms. Springer Netherlands; Dordrecht: 2010.
- Strandh S. A history of the machine. A & W Publ; New York: 1979.
-
- Ashton TS, Hudson P. The industrial revolution, 1760–1830. Oxford University Press; Oxford: 1997.
- Hudson P. The industrial revolution. Arnold; London: 2005.
- Berg M. The age of manufactures, 1700–1820. Industry, innovation and work in Britain. Routledge; London: 1996.
-
- Nicolaou KC, Sorensen EJ. Classics in Total Synthesis. Targets, strategies, methods. WILEY-VCH; Weinheim: 1996.
- Nicolaou KC, Snyder SA. Classics in total synthesis II. More targets, strategies, methods. WILEY-VCH; Weinheim: 2003.
- Nicolaou KC, Chen JS. Classics in total synthesis III. Further Targets, Strategies, Methods. WILEY-VCH; Weinheim: 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

