Sodium nitrate supplementation alters mitochondrial H2O2 emission but does not improve mitochondrial oxidative metabolism in the heart of healthy rats
- PMID: 29513565
- PMCID: PMC6139613
- DOI: 10.1152/ajpregu.00275.2017
Sodium nitrate supplementation alters mitochondrial H2O2 emission but does not improve mitochondrial oxidative metabolism in the heart of healthy rats
Abstract
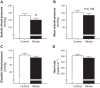
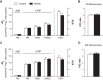
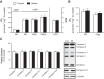
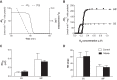
Supplementation with dietary inorganic nitrate ([Formula: see text]) is increasingly recognized to confer cardioprotective effects in both healthy and clinical populations. While the mechanism(s) remains ambiguous, in skeletal muscle oral consumption of NaNO3 has been shown to improve mitochondrial efficiency. Whether NaNO3 has similar effects on mitochondria within the heart is unknown. Therefore, we comprehensively investigated the effect of NaNO3 supplementation on in vivo left ventricular (LV) function and mitochondrial bioenergetics. Healthy male Sprague-Dawley rats were supplemented with NaNO3 (1 g/l) in their drinking water for 7 days. Echocardiography and invasive hemodynamics were used to assess LV morphology and function. Blood pressure (BP) was measured by tail-cuff and invasive hemodynamics. Mitochondrial bioenergetics were measured in LV isolated mitochondria and permeabilized muscle fibers by high-resolution respirometry and fluorometry. Nitrate decreased ( P < 0.05) BP, LV end-diastolic pressure, and maximal LV pressure. Rates of LV relaxation (when normalized to mean arterial pressure) tended ( P = 0.13) to be higher with nitrate supplementation. However, nitrate did not alter LV mitochondrial respiration, coupling efficiency, or oxygen affinity in isolated mitochondria or permeabilized muscle fibers. In contrast, nitrate increased ( P < 0.05) the propensity for mitochondrial H2O2 emission in the absence of changes in cellular redox state and decreased the sensitivity of mitochondria to ADP (apparent Km). These results add to the therapeutic potential of nitrate supplementation in cardiovascular diseases and suggest that nitrate may confer these beneficial effects via mitochondrial redox signaling.
Keywords: bioenergetics; heart; hemodynamics; mitochondria; nitrate.
Figures



Similar articles
-
Xanthine oxidase inhibition preserves left ventricular systolic but not diastolic function in cardiac volume overload.Am J Physiol Heart Circ Physiol. 2013 Nov 15;305(10):H1440-50. doi: 10.1152/ajpheart.00007.2013. Epub 2013 Sep 6. Am J Physiol Heart Circ Physiol. 2013. PMID: 24014679 Free PMC article.
-
Beetroot juice supplementation reduces whole body oxygen consumption but does not improve indices of mitochondrial efficiency in human skeletal muscle.J Physiol. 2016 Jan 15;594(2):421-35. doi: 10.1113/JP270844. Epub 2015 Dec 16. J Physiol. 2016. PMID: 26457670 Free PMC article.
-
Dietary nitrate increases submaximal SERCA activity and ADP transfer to mitochondria in slow-twitch muscle of female mice.Am J Physiol Endocrinol Metab. 2022 Aug 1;323(2):E171-E184. doi: 10.1152/ajpendo.00371.2021. Epub 2022 Jun 22. Am J Physiol Endocrinol Metab. 2022. PMID: 35732003
-
Mitochondrial Bioenergetics During Ischemia and Reperfusion.Adv Exp Med Biol. 2017;982:141-167. doi: 10.1007/978-3-319-55330-6_8. Adv Exp Med Biol. 2017. PMID: 28551786 Review.
-
Mitochondrial bioenergetics and cardiolipin alterations in myocardial ischemia-reperfusion injury: implications for pharmacological cardioprotection.Am J Physiol Heart Circ Physiol. 2018 Nov 1;315(5):H1341-H1352. doi: 10.1152/ajpheart.00028.2018. Epub 2018 Aug 10. Am J Physiol Heart Circ Physiol. 2018. PMID: 30095969 Review.
Cited by
-
Nitrite lowers the oxygen cost of ATP supply in cultured skeletal muscle cells by stimulating the rate of glycolytic ATP synthesis.PLoS One. 2022 Aug 8;17(8):e0266905. doi: 10.1371/journal.pone.0266905. eCollection 2022. PLoS One. 2022. PMID: 35939418 Free PMC article.
-
Dietary nitrate preserves mitochondrial bioenergetics and mitochondrial protein synthesis rates during short-term immobilization in mice.J Physiol. 2025 Jul;603(13):3795-3812. doi: 10.1113/JP284701. Epub 2023 Jun 28. J Physiol. 2025. PMID: 37293995 Free PMC article.
-
Sodium nitrate co-supplementation does not exacerbate low dose metronomic doxorubicin-induced cachexia in healthy mice.Sci Rep. 2020 Sep 24;10(1):15044. doi: 10.1038/s41598-020-71974-z. Sci Rep. 2020. PMID: 32973229 Free PMC article.
-
Inorganic nitrate and nitrite supplementation fails to improve skeletal muscle mitochondrial efficiency in mice and humans.Am J Clin Nutr. 2020 Jan 1;111(1):79-89. doi: 10.1093/ajcn/nqz245. Am J Clin Nutr. 2020. PMID: 31599928 Free PMC article.
References
-
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin C-T, Price JW 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009. doi:10.1172/JCI37048. - DOI - PMC - PubMed
-
- Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol (1985) 109: 135–148, 2010. doi:10.1152/japplphysiol.00046.2010. - DOI - PubMed
-
- Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol (1985) 107: 1144–1155, 2009. doi:10.1152/japplphysiol.00722.2009. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

