Liquid Biopsy in Head and Neck Cancer: Promises and Challenges
- PMID: 29513618
- PMCID: PMC5960882
- DOI: 10.1177/0022034518762071
Liquid Biopsy in Head and Neck Cancer: Promises and Challenges
Abstract
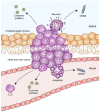
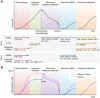
Head and neck cancer is the sixth most common cancer worldwide. It remains one of the leading causes of death, and its early detection is crucial. Liquid biopsy has emerged as a promising tool for detecting and monitoring the disease status of patients with early and advanced cancers. Circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and exosomal miRNAs have received enormous attention because of their apparent clinical implications. Analyses of these circulating biomarkers have paved the way for novel therapeutic approaches and precision medicine. A growing number of reports have implicated the use of circulating biomarkers for detection, treatment planning, response monitoring, and prognosis assessment. Although these new biomarkers can provide a wide range of possible clinical applications, no validated circulating biomarkers have yet been integrated into clinical practice for head and neck cancer. In this review, we summarize the current knowledge of circulating biomarkers in this field, focusing on their feasibility, limitations, and key areas of clinical applications. We also highlight recent advances in salivary diagnostics and their potential application in head and neck cancer.
Keywords: biomarker; circulating tumor DNA; circulating tumor cell; exosomal miRNA; saliva-exosomics; salivary diagnostics.
Conflict of interest statement
D.T.W. Wong is the cofounder of RNAmeTRIX, a molecular diagnostic company. D.T.W. Wong holds equity in RNAmeTRIX and serves as a company director and scientific advisor. The University of California also holds equity in RNAmeTRIX. Intellectual property that D.T.W. Wong invented and that was patented by the University of California has been licensed to RNAmeTRIX. D.T.W. Wong is a consultant to GlaxoSmithKlein, Wrigley, and Colgate-Palmolive. The authors declare no other potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures
References
-
- Alix-Panabières C, Pantel K. 2016. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 6(5):479–491. - PubMed
-
- Buglione M, Grisanti S, Almici C, Mangoni M, Polli C, Consoli F, Verardi R, Costa L, Paiar F, Pasinetti N, et al. 2012. Circulating tumour cells in locally advanced head and neck cancer: preliminary report about their possible role in predicting response to non-surgical treatment and survival. Eur J Cancer. 48(16):3019–3026. - PubMed
-
- Butt AQ, Mills KH. 2014. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene. 33(38):4623–4631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

