Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults
- PMID: 29513649
- PMCID: PMC6179355
- DOI: 10.1038/nature25975
Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults
Abstract
New neurons continue to be generated in the subgranular zone of the dentate gyrus of the adult mammalian hippocampus. This process has been linked to learning and memory, stress and exercise, and is thought to be altered in neurological disease. In humans, some studies have suggested that hundreds of new neurons are added to the adult dentate gyrus every day, whereas other studies find many fewer putative new neurons. Despite these discrepancies, it is generally believed that the adult human hippocampus continues to generate new neurons. Here we show that a defined population of progenitor cells does not coalesce in the subgranular zone during human fetal or postnatal development. We also find that the number of proliferating progenitors and young neurons in the dentate gyrus declines sharply during the first year of life and only a few isolated young neurons are observed by 7 and 13 years of age. In adult patients with epilepsy and healthy adults (18-77 years; n = 17 post-mortem samples from controls; n = 12 surgical resection samples from patients with epilepsy), young neurons were not detected in the dentate gyrus. In the monkey (Macaca mulatta) hippocampus, proliferation of neurons in the subgranular zone was found in early postnatal life, but this diminished during juvenile development as neurogenesis decreased. We conclude that recruitment of young neurons to the primate hippocampus decreases rapidly during the first years of life, and that neurogenesis in the dentate gyrus does not continue, or is extremely rare, in adult humans. The early decline in hippocampal neurogenesis raises questions about how the function of the dentate gyrus differs between humans and other species in which adult hippocampal neurogenesis is preserved.
Conflict of interest statement
Figures



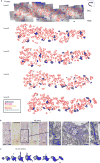


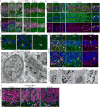







Comment in
-
Questioning human neurogenesis.Nature. 2018 Mar 15;555(7696):315-316. doi: 10.1038/d41586-018-02629-3. Nature. 2018. PMID: 29542697 No abstract available.
-
Neurogenesis: News of no new neurons?Nat Rev Neurosci. 2018 May;19(5):252-253. doi: 10.1038/nrn.2018.34. Epub 2018 Apr 5. Nat Rev Neurosci. 2018. PMID: 29618803 No abstract available.
-
Adult Human Hippocampal Neurogenesis: Controversy and Evidence.Trends Mol Med. 2018 Jun;24(6):521-522. doi: 10.1016/j.molmed.2018.04.002. Epub 2018 Apr 23. Trends Mol Med. 2018. PMID: 29699864
-
Letter: Human Hippocampal Neurogenesis Drops Sharply in Children to Undetectable Levels in Adults.Neurosurgery. 2018 Sep 1;83(3):E133-E137. doi: 10.1093/neuros/nyy252. Neurosurgery. 2018. PMID: 29873751 No abstract available.
-
Adult Neurogenesis in the Human Brain: Paradise Lost?Epilepsy Curr. 2018 Sep-Oct;18(5):329-331. doi: 10.5698/1535-7597.18.5.329. Epilepsy Curr. 2018. PMID: 30464737 Free PMC article. No abstract available.
-
Limits to human neurogenesis-really?Mol Psychiatry. 2020 Oct;25(10):2207-2209. doi: 10.1038/s41380-018-0337-5. Epub 2019 Jan 7. Mol Psychiatry. 2020. PMID: 30617274 Free PMC article. No abstract available.
-
Debate about birth of new neurons in adult brains extends to Alzheimer's disease.Nature. 2019 Mar;567(7749):433. doi: 10.1038/d41586-019-00891-7. Nature. 2019. PMID: 30911155 No abstract available.
-
Recalibrating the Existence of New Neurons in Adult Brain.ACS Chem Neurosci. 2019 May 15;10(5):2091-2093. doi: 10.1021/acschemneuro.9b00196. Epub 2019 Apr 22. ACS Chem Neurosci. 2019. PMID: 31007011
-
The Neuroscientist Comments.Neuroscientist. 2019 Aug;25(4):285. doi: 10.1177/1073858419859505. Neuroscientist. 2019. PMID: 31514645 No abstract available.
References
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed

