Why do open-label placebos work? A randomized controlled trial of an open-label placebo induction with and without extended information about the placebo effect in allergic rhinitis
- PMID: 29513699
- PMCID: PMC5841659
- DOI: 10.1371/journal.pone.0192758
Why do open-label placebos work? A randomized controlled trial of an open-label placebo induction with and without extended information about the placebo effect in allergic rhinitis
Abstract
Background: Several studies demonstrated that placebo treatment may have a significant impact on many different symptoms. While in the traditional view concealment of the placebo is essential, recent studies report intriguing evidence that placebos may work even without deception. For example, it has been demonstrated that open-label placebos can improve symptoms in allergic rhinitis. However, the mechanisms of how placebos without concealment work remain unknown.
Trial design: In order to examine expectancy effects we conducted a randomized controlled trial (N = 46), in which patients with allergic symptoms received either placebos without deception or no pills at all. In half of those patients we induced positive expectations about the placebo effect. After two weeks we tested whether symptoms and quality of life had changed.
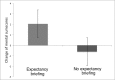
Results: Results revealed that open-label placebos improved allergic symptoms more than the control group. Inducing positive expectations had no effects on the improvement of allergic symptoms (the primary and more objective outcome), but on mental sum scores of the quality of life questionnaire.
Conclusions: Placebos without deception can improve symptoms in allergic rhinitis. Positive expectations do not contribute to the efficacy of open-label placebos, but seem to have an effect on more global and subjective well-being (mental or emotional quality of life).
Clinical trial registration number: German Clinical Trials Register, DRKS00012303.
Conflict of interest statement
Figures




References
-
- Kay AB. Overview of 'allergy and allergic diseases: with a view to the future'. British medical bulletin. 2000;56(4):843–64. Epub 2001/05/22. . - PubMed
-
- Wheatley LM, Togias A. Clinical practice. Allergic rhinitis. The New England journal of medicine. 2015;372(5):456–63. Epub 2015/01/30. doi: 10.1056/NEJMcp1412282 ; PubMed Central PMCID: PMCPMC4324099. - DOI - PMC - PubMed
-
- Abramson HA. Psychic factors in allergy and their treatment. Annals of allergy. 1956;14(2):145–51. Epub 1956/03/01. . - PubMed
-
- Czubalski K, Zawisza E. The role of psychic factors in patients with allergic rhinitis. Acta oto-laryngologica. 1976;81(5–6):484–8. Epub 1976/05/01. . - PubMed
-
- del Cuvillo A, Sastre J, Bartra J, Mullol J, DaVila I, Montoro J, et al. Placebo effect in clinical trials involving patients with allergic rhinitis. Journal of investigational allergology & clinical immunology. 2011;21 Suppl 3:40–5. Epub 2011/12/22. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

