A novel carbon tipped single micro-optrode for combined optogenetics and electrophysiology
- PMID: 29513711
- PMCID: PMC5841794
- DOI: 10.1371/journal.pone.0193836
A novel carbon tipped single micro-optrode for combined optogenetics and electrophysiology
Abstract
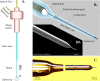
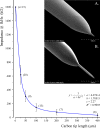
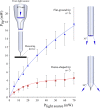
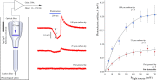
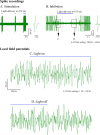
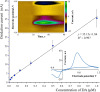
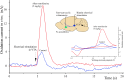
Optical microelectrodes (optrodes) are used in neuroscience to transmit light into the brain of a genetically modified animal to evoke and record electrical activity from light-sensitive neurons. Our novel micro-optrode solution integrates a light-transmitting 125 micrometer optical fiber and a 9 micrometer carbon monofilament to form an electrical lead element, which is contained in a borosilicate glass sheathing coaxial arrangement ending with a micrometer-sized carbon tip. This novel unit design is stiff and slender enough to be used for targeting deep brain areas, and may cause less tissue damage compared with previous models. The center-positioned carbon fiber is less prone to light-induced artifacts than side-lit metal microelectrodes previously presented. The carbon tip is capable of not only recording electrical signals of neuronal origin but can also provide valuable surface area for electron transfer, which is essential in electrochemical (voltammetry, amperometry) or microbiosensor applications. We present details of design and manufacture as well as operational examples of the newly developed single micro-optrode, which includes assessments of 1) carbon tip length-impedance relationship, 2) light transmission capabilities, 3) photoelectric artifacts in carbon fibers, 4) responses to dopamine using fast-scan cyclic voltammetry in vivo, and 5) optogenetic stimulation and spike or local field potential recording from the rat brain transfected with channelrhodopsin-2. With this work, we demonstrate that our novel carbon tipped single micro-optrode may open up new avenues for use in optogenetic stimulation when needing to be combined with extracellular recording, electrochemical, or microbiosensor measurements performed on a millisecond basis.
Conflict of interest statement
Figures


Similar articles
-
A fiber-based implantable multi-optrode array with contiguous optical and electrical sites.J Neural Eng. 2013 Aug;10(4):046020. doi: 10.1088/1741-2560/10/4/046020. Epub 2013 Jul 24. J Neural Eng. 2013. PMID: 23883568
-
A glass-coated tungsten microelectrode enclosing optical fibers for optogenetic exploration in primate deep brain structures.J Neurosci Methods. 2012 Oct 15;211(1):49-57. doi: 10.1016/j.jneumeth.2012.08.004. Epub 2012 Aug 14. J Neurosci Methods. 2012. PMID: 22971353
-
An integrated μLED optrode for optogenetic stimulation and electrical recording.IEEE Trans Biomed Eng. 2013 Jan;60(1):225-9. doi: 10.1109/TBME.2012.2217395. Epub 2012 Sep 6. IEEE Trans Biomed Eng. 2013. PMID: 22968201
-
Implantable optoelectronic probes for in vivo optogenetics.J Neural Eng. 2017 Jun;14(3):031001. doi: 10.1088/1741-2552/aa60b3. Epub 2017 Feb 15. J Neural Eng. 2017. PMID: 28198703 Review.
-
The fiber-optic imaging and manipulation of neural activity during animal behavior.Neurosci Res. 2016 Feb;103:1-9. doi: 10.1016/j.neures.2015.09.004. Epub 2015 Sep 30. Neurosci Res. 2016. PMID: 26427958 Review.
Cited by
-
Development of a Smart Wireless Multisensor Platform for an Optogenetic Brain Implant.Sensors (Basel). 2024 Jan 16;24(2):0. doi: 10.3390/s24020575. Sensors (Basel). 2024. PMID: 38257668 Free PMC article.
-
Flexible Neural Probes with Electrochemical Modified Microelectrodes for Artifact-Free Optogenetic Applications.Int J Mol Sci. 2021 Oct 26;22(21):11528. doi: 10.3390/ijms222111528. Int J Mol Sci. 2021. PMID: 34768957 Free PMC article.
-
A novel low-cost electrode for recording the local field potential of freely moving rat's brain.Transl Neurosci. 2020 Jun 5;11(1):96-104. doi: 10.1515/tnsci-2020-0104. eCollection 2020. Transl Neurosci. 2020. PMID: 33312716 Free PMC article.
-
Multifunctional and Flexible Neural Probe with Thermally Drawn Fibers for Bidirectional Synaptic Probing in the Brain.ACS Nano. 2024 May 21;18(20):13277-13285. doi: 10.1021/acsnano.4c02578. Epub 2024 May 10. ACS Nano. 2024. PMID: 38728175 Free PMC article.
-
Artifact-free and high-temporal-resolution in vivo opto-electrophysiology with microLED optoelectrodes.Nat Commun. 2020 Apr 28;11(1):2063. doi: 10.1038/s41467-020-15769-w. Nat Commun. 2020. PMID: 32345971 Free PMC article.
References
-
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–8. doi: 10.1038/nn1525 . - DOI - PubMed
-
- Deisseroth K. Optogenetics. Nat Methods. 2011;8(1):26–9. doi: 10.1038/nmeth.f.324 . - DOI - PMC - PubMed
-
- Dufour S, De Koninck Y. Optrodes for combined optogenetics and electrophysiology in live animals. Neurophotonics. 2015;2(3):031205 doi: 10.1117/1.NPh.2.3.031205 ; PubMed Central PMCID: PMCPMC4489589. - DOI - PMC - PubMed
-
- Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, et al. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci. 2007;27(52):14231–8. doi: 10.1523/JNEUROSCI.3578-07.2007 . - DOI - PMC - PubMed
-
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458(7241):1025–9. doi: 10.1038/nature07926 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

