In ovo CpG DNA delivery increases innate and adaptive immune cells in respiratory, gastrointestinal and immune systems post-hatch correlating with lower infectious laryngotracheitis virus infection
- PMID: 29513732
- PMCID: PMC5841808
- DOI: 10.1371/journal.pone.0193964
In ovo CpG DNA delivery increases innate and adaptive immune cells in respiratory, gastrointestinal and immune systems post-hatch correlating with lower infectious laryngotracheitis virus infection
Abstract
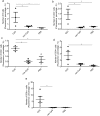
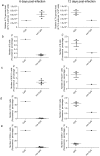
Cytosine-guanosine deoxynucleotides (CpG) DNA can be delivered in ovo at embryo day (ED)18 for the stimulation of toll-like receptor (TLR)21 signaling pathway that ultimately protects chickens against a number of bacterial and viral infections. There is a dearth of information understanding the mechanisms of protection induced by in ovo delivered CpG DNA. The objective of this study was to determine the immune cell changes post-hatch following in ovo delivery of the TLR21 ligand, CpG DNA. In order to quantify changes of percentage of KUL01+, IgM+ B, cluster of differentiation (CD)4+ and CD8α+ cells, trachea, lung, duodenum, large intestine, spleen and bursa of Fabricius were collected on day 1 post-hatch. We found increased recruitments of KUL01+ cells, in organs of these body systems post-hatch following in ovo delivery of CpG DNA. Although IgM+ B cells, CD4+ and CD8α+ cells were increased in lungs and immune system organs, these cells were not quantifiable from the trachea, duodenum and large intestine immediately following the hatch. Furthermore, when CpG DNA is delivered in ovo and subsequently infected with infectious laryngotracheitis virus (ILTV) post-hatch on day 1, CpG DNA reduces morbidity and mortality resulting from ILTV infection. This study provides insights into the mechanisms of host responses elicited following in ovo delivery of CpG DNA in avian species.
Conflict of interest statement
Figures



Similar articles
-
In ovo delivery of CpG DNA reduces avian infectious laryngotracheitis virus induced mortality and morbidity.Viruses. 2015 Apr 8;7(4):1832-52. doi: 10.3390/v7041832. Viruses. 2015. PMID: 25856635 Free PMC article.
-
In ovo delivery of Toll-like receptor 2 ligand, lipoteichoic acid induces pro-inflammatory mediators reducing post-hatch infectious laryngotracheitis virus infection.Vet Immunol Immunopathol. 2015 Apr 15;164(3-4):170-8. doi: 10.1016/j.vetimm.2015.02.006. Epub 2015 Feb 20. Vet Immunol Immunopathol. 2015. PMID: 25764942
-
Replication of recombinant herpesvirus of turkey expressing genes of infectious laryngotracheitis virus in specific pathogen free and broiler chickens following in ovo and subcutaneous vaccination.Avian Pathol. 2011 Aug;40(4):395-403. doi: 10.1080/03079457.2011.588196. Avian Pathol. 2011. PMID: 21812719
-
Immune responses to infectious laryngotracheitis virus.Dev Comp Immunol. 2013 Nov;41(3):454-62. doi: 10.1016/j.dci.2013.03.022. Epub 2013 Apr 6. Dev Comp Immunol. 2013. PMID: 23567343 Review.
-
Comparison of in ovo and post-hatch vaccination with particular reference to infectious bursal disease. A review.Vet Q. 2004 Jun;26(2):76-87. doi: 10.1080/01652176.2004.9695170. Vet Q. 2004. PMID: 15230052 Review.
Cited by
-
Single stranded (ss)RNA-mediated antiviral response against infectious laryngotracheitis virus infection.BMC Microbiol. 2019 Feb 8;19(1):34. doi: 10.1186/s12866-019-1398-6. BMC Microbiol. 2019. PMID: 30736730 Free PMC article.
-
Antiviral response elicited against avian influenza virus infection following activation of toll-like receptor (TLR)7 signaling pathway is attributable to interleukin (IL)-1β production.BMC Res Notes. 2018 Dec 4;11(1):859. doi: 10.1186/s13104-018-3975-4. BMC Res Notes. 2018. PMID: 30514372 Free PMC article.
-
Recent advances in delivery of veterinary DNA vaccines against avian pathogens.Vet Res. 2019 Oct 10;50(1):78. doi: 10.1186/s13567-019-0698-z. Vet Res. 2019. PMID: 31601266 Free PMC article. Review.
-
The In Ovo Delivery of CpG Oligonucleotides Protects against Infectious Bronchitis with the Recruitment of Immune Cells into the Respiratory Tract of Chickens.Viruses. 2018 Nov 15;10(11):635. doi: 10.3390/v10110635. Viruses. 2018. PMID: 30445707 Free PMC article.
-
Avian Pattern Recognition Receptor Sensing and Signaling.Vet Sci. 2020 Jan 27;7(1):14. doi: 10.3390/vetsci7010014. Vet Sci. 2020. PMID: 32012730 Free PMC article. Review.
References
-
- Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert review of vaccines. 2011;10(4):499–511. Epub 2011/04/22. doi: 10.1586/erv.10.174 . - DOI - PMC - PubMed
-
- Dalpke AH, Heeg K. CpG-DNA as immune response modifier. International journal of medical microbiology: IJMM. 2004;294(5):345–54. Epub 2004/11/10. doi: 10.1016/j.ijmm.2004.07.005 . - DOI - PubMed
-
- Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nature reviews Drug discovery. 2006;5(6):471–84. Epub 2006/06/10. doi: 10.1038/nrd2059 . - DOI - PubMed
-
- Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–9. Epub 1995/04/06. doi: 10.1038/374546a0 . - DOI - PubMed
-
- Brownlie R, Zhu J, Allan B, Mutwiri GK, Babiuk LA, Potter A, et al. Chicken TLR21 acts as a functional homologue to mammalian TLR9 in the recognition of CpG oligodeoxynucleotides. Molecular immunology. 2009;46(15):3163–70. Epub 2009/07/04. doi: 10.1016/j.molimm.2009.06.002 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

