Identification of a novel UMOD mutation (c.163G>A) in a Brazilian family with autosomal dominant tubulointerstitial kidney disease
- PMID: 29513881
- PMCID: PMC5912098
- DOI: 10.1590/1414-431X20176560
Identification of a novel UMOD mutation (c.163G>A) in a Brazilian family with autosomal dominant tubulointerstitial kidney disease
Abstract
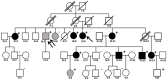
Autosomal dominant tubulointerstitial kidney disease (ADTKD) is characterized by autosomal dominant inheritance, progressive chronic kidney disease, and a bland urinary sediment. ADTKD is most commonly caused by mutations in the UMOD gene encoding uromodulin (ADTKD-UMOD). We herein report the first confirmed case of a multi-generational Brazilian family with ADTKD-UMOD, caused by a novel heterozygous mutation (c.163G>A, GGC→AGC, p.Gly55Ser) in the UMOD gene. Of 41 family members, 22 underwent genetic analysis, with 11 individuals found to have this mutation. Three affected individuals underwent hemodialysis, one peritoneal dialysis, and one patient received a kidney transplant from a family member later found to be genetically affected. Several younger individuals affected with the mutation were also identified. Clinical characteristics included a bland urinary sediment in all tested individuals and a kidney biopsy in one individual showing tubulointerstitial fibrosis. Unlike most other reported families with ADTKD-UMOD, neither gout nor hyperuricemia was found in affected individuals. In summary, we report a novel UMOD mutation in a Brazilian family with 11 affected members, and we discuss the importance of performing genetic testing in families with inherited kidney disease of unknown cause.
Figures


References
-
- Bolar NA, Golzio C, Zivna M, Hayot G, Van Hemelrijk C, Schepers D, et al. Heterozygous loss-of-function SEC61A1 Mutations cause autosomal-dominant tubulo-interstitial and glomerulocystic kidney disease with anemia. Am J Hum Genet. 2016;99:174–187. doi: 10.1016/j.ajhg.2016.05.028. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

