The Pericyte of the Pancreatic Islet Regulates Capillary Diameter and Local Blood Flow
- PMID: 29514070
- PMCID: PMC5876933
- DOI: 10.1016/j.cmet.2018.02.016
The Pericyte of the Pancreatic Islet Regulates Capillary Diameter and Local Blood Flow
Abstract
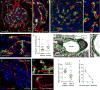
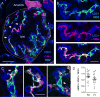
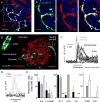
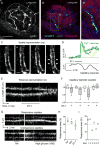
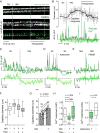
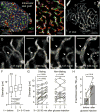
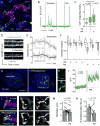
Efficient insulin secretion requires a well-functioning pancreatic islet microvasculature. The dense network of islet capillaries includes the islet pericyte, a cell that has barely been studied. Here we show that islet pericytes help control local blood flow by adjusting islet capillary diameter. Islet pericytes cover 40% of the microvasculature, are contractile, and are innervated by sympathetic axons. Sympathetic adrenergic input increases pericyte activity and reduces capillary diameter and local blood flow. By contrast, activating beta cells by increasing glucose concentration inhibits pericytes, dilates islet capillaries, and increases local blood flow. These effects on pericytes are mediated by endogenous adenosine, which is likely derived from ATP co-released with insulin. Pericyte coverage of islet capillaries drops drastically in type 2 diabetes, suggesting that, under diabetic conditions, islets lose this mechanism to control their own blood supply. This may lead to inadequate insulin release into the circulation, further deteriorating glycemic control.
Keywords: adenosine; blood flow; capillary diameter; diabetes; hyperemia; insulin secretion; pancreatic islet; pancreatic slice; pericytes; sympathetic.
Published by Elsevier Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. - PubMed
-
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. - PubMed
-
- Atef N, Ktorza A, Picon L, Penicaud L. Increased islet blood flow in obese rats: role of the autonomic nervous system. Am J Physiol. 1992;262:E736–740. - PubMed
-
- Ballian N, Brunicardi FC. Islet vasculature as a regulator of endocrine pancreas function. World J Surg. 2007;31:705–714. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

