Estrogen Deficiency Promotes Hepatic Steatosis via a Glucocorticoid Receptor-Dependent Mechanism in Mice
- PMID: 29514097
- PMCID: PMC5875726
- DOI: 10.1016/j.celrep.2018.02.041
Estrogen Deficiency Promotes Hepatic Steatosis via a Glucocorticoid Receptor-Dependent Mechanism in Mice
Abstract
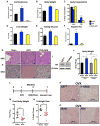
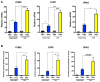
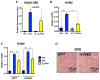
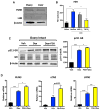
Glucocorticoids (GCs) are master regulators of systemic metabolism. Intriguingly, Cushing's syndrome, a disorder of excessive GCs, phenocopies several menopause-induced metabolic pathologies. Here, we show that the glucocorticoid receptor (GR) drives steatosis in hypogonadal female mice because hepatocyte-specific GR knockout mice are refractory to developing ovariectomy-induced steatosis. Intriguingly, transcriptional profiling revealed that ovariectomy elicits hepatic GC hypersensitivity globally. Hypogonadism-induced GC hypersensitivity results from a loss of systemic but not hepatic estrogen (E2) signaling, given that hepatocyte-specific E2 receptor deletion does not confer GC hypersensitivity. Mechanistically, enhanced chromatin recruitment and ligand-dependent hyperphosphorylation of GR underlie ovariectomy-induced glucocorticoid hypersensitivity. The dysregulated glucocorticoid-mediated signaling present in hypogonadal females is a product of increased follicle-stimulating hormone (FSH) production because FSH treatment in ovary-intact mice recapitulates glucocorticoid hypersensitivity similar to hypogonadal female mice. Our findings uncover a regulatory axis between estradiol, FSH, and hepatic glucocorticoid receptor signaling that, when disrupted, as in menopause, promotes hepatic steatosis.
Keywords: estrogen receptor; follicle-stimulating hormone; glucocorticoid receptor; lipid metabolism; liver; menopause; metabolic syndrome; steatosis.
Published by Elsevier Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Impairment of hepatic growth hormone and glucocorticoid receptor signaling causes steatosis and hepatocellular carcinoma in mice.Hepatology. 2011 Oct;54(4):1398-409. doi: 10.1002/hep.24509. Hepatology. 2011. PMID: 21725989 Free PMC article.
-
AMPK regulates metabolic actions of glucocorticoids by phosphorylating the glucocorticoid receptor through p38 MAPK.Mol Endocrinol. 2010 Sep;24(9):1748-64. doi: 10.1210/me.2010-0192. Epub 2010 Jul 21. Mol Endocrinol. 2010. PMID: 20660302 Free PMC article.
-
Glucocorticoid Receptor β Induces Hepatic Steatosis by Augmenting Inflammation and Inhibition of the Peroxisome Proliferator-activated Receptor (PPAR) α.J Biol Chem. 2016 Dec 9;291(50):25776-25788. doi: 10.1074/jbc.M116.752311. Epub 2016 Oct 26. J Biol Chem. 2016. PMID: 27784782 Free PMC article.
-
Hepatic growth hormone and glucocorticoid receptor signaling in body growth, steatosis and metabolic liver cancer development.Mol Cell Endocrinol. 2012 Sep 25;361(1-2):1-11. doi: 10.1016/j.mce.2012.03.026. Epub 2012 Apr 30. Mol Cell Endocrinol. 2012. PMID: 22564914 Free PMC article. Review.
-
The glucocorticoid receptor: cause of or cure for obesity?Am J Physiol Endocrinol Metab. 2016 Feb 15;310(4):E249-57. doi: 10.1152/ajpendo.00478.2015. Epub 2015 Dec 29. Am J Physiol Endocrinol Metab. 2016. PMID: 26714851 Free PMC article. Review.
Cited by
-
The importance of estradiol for body weight regulation in women.Front Endocrinol (Lausanne). 2022 Nov 7;13:951186. doi: 10.3389/fendo.2022.951186. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36419765 Free PMC article. Review.
-
Change in metabolic parameters and reproductive hormones from baseline to 6-month hormone therapy.Medicine (Baltimore). 2022 Jan 7;101(1):e28361. doi: 10.1097/MD.0000000000028361. Medicine (Baltimore). 2022. PMID: 35029882 Free PMC article.
-
The glucocorticoid receptor agonistic modulators CpdX and CpdX-D3 do not generate the debilitating effects of synthetic glucocorticoids.Proc Natl Acad Sci U S A. 2019 Jul 9;116(28):14200-14209. doi: 10.1073/pnas.1908264116. Epub 2019 Jun 20. Proc Natl Acad Sci U S A. 2019. PMID: 31221758 Free PMC article.
-
The role of hepatic sinusoidal microenvironment in NASH: pathogenesis, animal models, and therapeutic prospects.Front Pharmacol. 2025 Apr 28;16:1467950. doi: 10.3389/fphar.2025.1467950. eCollection 2025. Front Pharmacol. 2025. PMID: 40356963 Free PMC article. Review.
-
A Body Shape Index (ABSI), hip index, and risk of cancer in the UK Biobank cohort.Cancer Med. 2021 Aug;10(16):5614-5628. doi: 10.1002/cam4.4097. Epub 2021 Jul 1. Cancer Med. 2021. PMID: 34196490 Free PMC article.
References
-
- Arnaldi G, Scandali VM, Trementino L, Cardinaletti M, Appolloni G, Boscaro M. Pathophysiology of dyslipidemia in Cushing’s syndrome. Neuroendocrinology. 2010;92(Suppl 1):86–90. - PubMed
-
- Bingol B, Gunenc Z, Yilmaz M, Biri A, Tiras B, Güner H. Effects of hormone replacement therapy on glucose and lipid profiles and on cardiovascular risk parameters in postmenopausal women. Arch Gynecol Obstet. 2010;281:857–864. - PubMed
-
- Bougarne N, Paumelle R, Caron S, Hennuyer N, Mansouri R, Gervois P, Staels B, Haegeman G, De Bosscher K. PPARalpha blocks glucocorticoid receptor alpha-mediated transactivation but cooperates with the activated glucocorticoid receptor alpha for transrepression on NF-kappaB. Proc Natl Acad Sci USA. 2009;106:7397–7402. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

