Late Presentation With HIV in Africa: Phenotypes, Risk, and Risk Stratification in the REALITY Trial
- PMID: 29514235
- PMCID: PMC5850547
- DOI: 10.1093/cid/cix1142
Late Presentation With HIV in Africa: Phenotypes, Risk, and Risk Stratification in the REALITY Trial
Abstract
Background: Severely immunocompromised human immunodeficiency virus (HIV)-infected individuals have high mortality shortly after starting antiretroviral therapy (ART). We investigated predictors of early mortality and "late presenter" phenotypes.
Methods: The Reduction of EArly MortaLITY (REALITY) trial enrolled ART-naive adults and children ≥5 years of age with CD4 counts <100 cells/µL initiating ART in Uganda, Zimbabwe, Malawi, and Kenya. Baseline predictors of mortality through 48 weeks were identified using Cox regression with backwards elimination (exit P > .1).
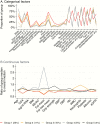
Results: Among 1711 included participants, 203 (12%) died. Mortality was independently higher with older age; lower CD4 count, albumin, hemoglobin, and grip strength; presence of World Health Organization stage 3/4 weight loss, fever, or vomiting; and problems with mobility or self-care at baseline (all P < .04). Receiving enhanced antimicrobial prophylaxis independently reduced mortality (P = .02). Of five late-presenter phenotypes, Group 1 (n = 355) had highest mortality (25%; median CD4 count, 28 cells/µL), with high symptom burden, weight loss, poor mobility, and low albumin and hemoglobin. Group 2 (n = 394; 11% mortality; 43 cells/µL) also had weight loss, with high white cell, platelet, and neutrophil counts suggesting underlying inflammation/infection. Group 3 (n = 218; 10% mortality) had low CD4 counts (27 cells/µL), but low symptom burden and maintained fat mass. The remaining groups had 4%-6% mortality.
Conclusions: Clinical and laboratory features identified groups with highest mortality following ART initiation. A screening tool could identify patients with low CD4 counts for prioritizing same-day ART initiation, enhanced prophylaxis, and intensive follow-up.
Clinical trials registration: ISRCTN43622374.
Figures
References
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach 2nd ed 2016. Available at: http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed 13 January 2018.
-
- TEMPRANO ANRS Study Group , Danel C, Moh R, Gabillard D et al. . A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–22. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- MC_UU_12023/17/MRC_/Medical Research Council/United Kingdom
- MR/M007367/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12023/23/MRC_/Medical Research Council/United Kingdom
- MC_UU_12023/26/MRC_/Medical Research Council/United Kingdom
- MC_EX_G1100693/MRC_/Medical Research Council/United Kingdom
- U01 AI069911/AI/NIAID NIH HHS/United States
- 108065/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- 101113/Z/13/Z/WT_/Wellcome Trust/United Kingdom
- G1100693/MRC_/Medical Research Council/United Kingdom
- 203077/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- MC_EX_UU_G1100693/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MR/P022251/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

