Quantifying the Impact of Natural Immunity on Rotavirus Vaccine Efficacy Estimates: A Clinical Trial in Dhaka, Bangladesh (PROVIDE) and a Simulation Study
- PMID: 29514306
- PMCID: PMC5853827
- DOI: 10.1093/infdis/jix668
Quantifying the Impact of Natural Immunity on Rotavirus Vaccine Efficacy Estimates: A Clinical Trial in Dhaka, Bangladesh (PROVIDE) and a Simulation Study
Abstract
Background: The low efficacy of rotavirus vaccines in clinical trials performed in low-resource settings may be partially explained by acquired immunity from natural exposure, especially in settings with high disease incidence.
Methods: In a clinical trial of monovalent rotavirus vaccine in Bangladesh, we compared the original per-protocol efficacy estimate to efficacy derived from a recurrent events survival model in which children were considered naturally exposed and potentially immune after their first rotavirus diarrhea (RVD) episode. We then simulated trial cohorts to estimate the expected impact of prior exposure on efficacy estimates for varying rotavirus incidence rates and vaccine efficacies.
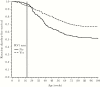
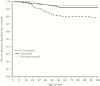
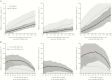
Results: Accounting for natural immunity increased the per-protocol vaccine efficacy estimate against severe RVD from 63.1% (95% confidence interval [CI], 33.0%-79.7%) to 70.2% (95% CI, 44.5%-84.0%) in the postvaccination period, and original year 2 efficacy was underestimated by 14%. The simulations demonstrated that this expected impact increases linearly with RVD incidence, will be greatest for vaccine efficacies near 50%, and can reach 20% in settings with high incidence and low efficacy.
Conclusions: High rotavirus incidence leads to predictably lower vaccine efficacy estimates due to the acquisition of natural immunity in unvaccinated children, and this phenomenon should be considered when comparing efficacy estimates across settings.
Clinical trials registration: NCT01375647.
Figures
Comment in
-
Waxing Understanding of Waning Immunity.J Infect Dis. 2018 Mar 5;217(6):851-853. doi: 10.1093/infdis/jix670. J Infect Dis. 2018. PMID: 29394364 Free PMC article. No abstract available.
References
-
- PATH Rotavirus vaccine country introductions: maps and list. PATH vaccine resource library. 2016. http://www.path.org/vaccineresources/details.php?i=2235. Accessed 9 March 2017.
-
- Patel MM, Glass R, Desai R, Tate JE, Parashar UD. Fulfilling the promise of rotavirus vaccines: how far have we come since licensure?Lancet Infect Dis 2012; 12:561–70. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

