Similar efficacy, safety and immunogenicity of adalimumab biosimilar BI 695501 and Humira reference product in patients with moderately to severely active rheumatoid arthritis: results from the phase III randomised VOLTAIRE-RA equivalence study
- PMID: 29514803
- PMCID: PMC5965346
- DOI: 10.1136/annrheumdis-2017-212245
Similar efficacy, safety and immunogenicity of adalimumab biosimilar BI 695501 and Humira reference product in patients with moderately to severely active rheumatoid arthritis: results from the phase III randomised VOLTAIRE-RA equivalence study
Abstract
Objective: To demonstrate clinical equivalence of adalimumab biosimilar candidate BI 695501 with Humira.
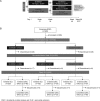
Methods: Patients with active rheumatoid arthritis on stable methotrexate were randomised to BI 695501 or Humira in a double-blind, parallel-group, equivalence study. At week 24, patients were rerandomised to continue BI 695501 or Humira, or switch from Humira to BI 695501. The coprimary endpoints were the percentage of patients achieving the American College of Rheumatology 20% response criteria (ACR20) at weeks 12 and 24. Further efficacy and safety endpoints and immunogenicity were assessed up to week 58.
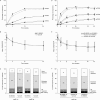
Results: 645 patients were randomised. At week 12, 67.0% and 61.1% (90% CI -0.9 to 12.7) of patients receiving BI 695501 (n=324) and Humira (n=321), respectively, achieved ACR20; at week 24 the corresponding values were 69.0% and 64.5% (95% CI -3.4 to 12.5). These differences were within prespecified margins (week 12: 90% CI (-12% to 15%); week 24: 95% CI (-15% to 15%)), demonstrating therapeutic bioequivalence. 593 patients were rerandomised at week 24. Up to week 48, mean change from baseline in Disease Activity Score 28-erythrocyte sedimentation rate and ACR20/ACR50/ACR70 response rates were similar across the switched (n=147), continuous BI 695501 (n=298) and continuous Humira (n=148) groups. Similar immunogenicity (antidrug antibodies (ADAs), ADA titres and neutralising antibodies) was seen between BI 695501 and Humira (to week 24) and across rerandomised groups (to week 48). Safety and tolerability profiles were similar between groups.
Conclusions: BI 695501 demonstrated similar efficacy, safety and immunogenicity to Humira; switch from Humira to BI 695501 had no impact on efficacy, safety and immunogenicity.
Trial registration number: NCT02137226, Results.
Keywords: anti-tnf; autoimmune diseases; dmards (biologic); rheumatoid arthritis; treatment.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: SBC and ECL received funding from Boehringer Ingelheim, study sponsor, as principal investigators of this study. AA-R and PAK have no competing interests to declare. NP, IS and DA are (or were) employees of Boehringer Ingelheim, study sponsor.
Figures

Comment in
-
Plain language summary of the VOLTAIRE-RA in patients with moderate-to-severe rheumatoid arthritis.Immunotherapy. 2022 Oct;14(15):1183-1190. doi: 10.2217/imt-2022-0106. Epub 2022 Sep 6. Immunotherapy. 2022. PMID: 36065786
References
-
- Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product. 2012. https://www.fda.gov/downloads/DrugsGuidanceComplianceRegulatoryInformati...
-
- European Medicines Agency. Guideline on similar biological medicinal products. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin...
-
- European Medicines Agency. Guideline on similar biological medicinal products containing monoclonal antibodies - non-clinical and clinical issues. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

