Beyond Competitive Inhibition: Regulation of ABC Transporters by Kinases and Protein-Protein Interactions as Potential Mechanisms of Drug-Drug Interactions
- PMID: 29514827
- PMCID: PMC5896366
- DOI: 10.1124/dmd.118.080663
Beyond Competitive Inhibition: Regulation of ABC Transporters by Kinases and Protein-Protein Interactions as Potential Mechanisms of Drug-Drug Interactions
Abstract
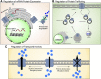
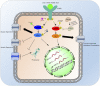
ATP-binding cassette (ABC) transporters are transmembrane efflux transporters mediating the extrusion of an array of substrates ranging from amino acids and lipids to xenobiotics, and many therapeutic compounds, including anticancer drugs. The ABC transporters are also recognized as important contributors to pharmacokinetics, especially in drug-drug interactions and adverse drug effects. Drugs and xenobiotics, as well as pathologic conditions, can influence the transcription of ABC transporters, or modify their activity or intracellular localization. Kinases can affect the aforementioned processes for ABC transporters as do protein interactions. In this review, we focus on the ABC transporters ABCB1, ABCB11, ABCC1, ABCC4, and ABCG2 and illustrate how kinases and protein-protein interactions affect these transporters. The clinical relevance of these factors is currently unknown; however, these examples suggest that our understanding of drug-drug interactions will benefit from further knowledge of how kinases and protein-protein interactions affect ABC transporters.
Copyright © 2018 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


References
-
- Adachi M, Sampath J, Lan LB, Sun D, Hargrove P, Flatley R, Tatum A, Edwards MZ, Wezeman M, Matherly L, et al. (2002) Expression of MRP4 confers resistance to ganciclovir and compromises bystander cell killing. J Biol Chem 277:38998–39004. - PubMed
-
- Aho TL, Sandholm J, Peltola KJ, Mankonen HP, Lilly M, Koskinen PJ. (2004) Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett 571:43–49. - PubMed
-
- Allen JD, Jackson SC, Schinkel AH. (2002) A mutation hot spot in the Bcrp1 (Abcg2) multidrug transporter in mouse cell lines selected for doxorubicin resistance. Cancer Res 62:2294–2299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

