Genome-wide Discovery and Identification of a Novel miRNA Signature for Recurrence Prediction in Stage II and III Colorectal Cancer
- PMID: 29514841
- PMCID: PMC6095767
- DOI: 10.1158/1078-0432.CCR-17-3236
Genome-wide Discovery and Identification of a Novel miRNA Signature for Recurrence Prediction in Stage II and III Colorectal Cancer
Abstract
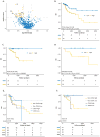
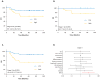
Purpose: The current tumor-node-metastasis (TNM) staging system is inadequate at identifying patients with high-risk colorectal cancer. Using a systematic and comprehensive biomarker discovery and validation approach, we aimed to identify an miRNA recurrence classifier (MRC) that can improve upon the current TNM staging as well as is superior to currently offered molecular assays.Experimental Design: Three independent genome-wide miRNA expression profiling datasets were used for biomarker discovery (N = 158) and in silico validation (N = 109 and N = 40) to identify an miRNA signature for predicting tumor recurrence in patients with colorectal cancer. Subsequently, this signature was analytically trained and validated in retrospectively collected independent patient cohorts of fresh-frozen (N = 127, cohort 1) and formalin-fixed paraffin-embedded (FFPE; N = 165, cohort 2 and N = 139, cohort 3) specimens.Results: We identified an 8-miRNA signature that significantly predicted recurrence-free interval (RFI) in the discovery (P = 0.002) and two independent publicly available datasets (P = 0.00006 and P = 0.002). The RT-PCR-based validation in independent clinical cohorts revealed that MRC-derived high-risk patients succumb to significantly poor RFI in patients with stage II and III colorectal cancer [cohort 1: hazard ratio (HR), 3.44 (1.56-7.45), P = 0.001; cohort 2: HR, 6.15 (3.33-11.35), P = 0.001; and cohort 3: HR, 4.23 (2.26-7.92), P = 0.0003]. In multivariate analyses, MRC emerged as an independent predictor of tumor recurrence and achieved superior predictive accuracy over the currently available molecular assays. The RT-PCR-based MRC risk score = (-0.1218 × miR-744) + (-3.7142 × miR-429) + (-2.2051 × miR-362) + (3.0564 × miR-200b) + (2.4997 × miR-191) + (-0.0065 × miR-30c2) + (2.2224 × miR-30b) + (-1.1162 × miR-33a).Conclusions: This novel MRC is superior to currently used clinicopathologic features, as well as National Comprehensive Cancer Network (NCCN) criteria, and works regardless of adjuvant chemotherapy status in identifying patients with high-risk stage II and III colorectal cancer. This can be readily deployed in clinical practice with FFPE specimens for decision-making pending further model testing and validation. Clin Cancer Res; 24(16); 3867-77. ©2018 AACRSee related commentary by Rodriguez et al., p. 3787.
©2018 American Association for Cancer Research.
Conflict of interest statement
The author shave declared no conflict of interest
Figures


Comment in
-
Adjuvant Therapy for Colon Cancer: Genes, Genes… and the Patient in the Center.Clin Cancer Res. 2018 Aug 15;24(16):3787-3789. doi: 10.1158/1078-0432.CCR-18-0818. Epub 2018 Apr 20. Clin Cancer Res. 2018. PMID: 29678903
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

