Transmembrane redox control and proteolysis of PdeC, a novel type of c-di-GMP phosphodiesterase
- PMID: 29514851
- PMCID: PMC5897775
- DOI: 10.15252/embj.201797825
Transmembrane redox control and proteolysis of PdeC, a novel type of c-di-GMP phosphodiesterase
Abstract
The nucleotide second messenger c-di-GMP nearly ubiquitously promotes bacterial biofilm formation, with enzymes that synthesize and degrade c-di-GMP being controlled by diverse N-terminal sensor domains. Here, we describe a novel class of widely occurring c-di-GMP phosphodiesterases (PDE) that feature a periplasmic "CSS domain" with two highly conserved cysteines that is flanked by two transmembrane regions (TM1 and TM2) and followed by a cytoplasmic EAL domain with PDE activity. Using PdeC, one of the five CSS domain PDEs of Escherichia coli K-12, we show that DsbA/DsbB-promoted disulfide bond formation in the CSS domain reduces PDE activity. By contrast, the free thiol form is enzymatically highly active, with the TM2 region promoting dimerization. Moreover, this form is processed by periplasmic proteases DegP and DegQ, yielding a highly active TM2 + EAL fragment that is slowly removed by further proteolysis. Similar redox control and proteolysis was also observed for a second CSS domain PDE, PdeB. At the physiological level, CSS domain PDEs modulate production and supracellular architecture of extracellular matrix polymers in the deeper layers of mature E. coli biofilms.
Keywords: DegP; DsbAB; EAL domain; biofilm; second messenger.
© 2018 The Authors. Published under the terms of the CC BY NC ND 4.0 license.
Figures

Macrocolonies of Escherichia coli K‐12 strain AR3110 expressing the indicated PdeC versions under control of the tac promoter from the low copy number plasmid pCAB18 were grown on salt‐free LB (LBnoS) or YESCA agar plates supplemented with the matrix dye Congo red (CR) for 5 days at 28°C. For comparison with morphological patterns of macrocolonies of standard strains, see Appendix Fig S1.
Derivatives of strain W3110 carrying a single copy csgB::lacZ reporter fusion and containing the indicated plasmids were grown in LB medium. Samples were taken during the growth of the cultures (numbers refer to hours of growth and respond to data sampling and measurement points shown in C; on, overnight). Cellular CsgD levels were determined by immunoblotting.
From the same samples as in (B), specific β‐galactosidase activities expressed from csgB::lacZ were determined.
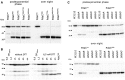
Derivatives of strain W3110 containing the indicated plasmids were grown in LB medium, and samples were taken during post‐exponential growth and from overnight (“on”) cultures. PdeC variants (containing C‐terminal His6 tags) produced from the indicated plasmids were detected by immunoblotting.
Strain W3110 containing the PdeCwt plasmid was grown to an OD578 of 1.2 and 6.5 mM DTT was added where indicated, with samples for immunoblotting taken at the times indicated. As a reference for the 33‐kD proteolytic fragment of PdeC, a sample obtained from the PdeCASS plasmid strain after overnight growth was included (last lane).
Derivatives of strain W3110 carrying the indicated mutations in the different dsb genes and containing the plasmids expressing either PdeCwt or PdeCASS were grown in LB medium. Samples were taken during post‐exponential growth and from overnight cultures and PdeC variants expressed were visualized by immunoblotting.
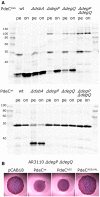
Derivatives of strain W3110 carrying single or double mutations in degP and degQ and containing the plasmids expressing either PdeCwt or PdeCASS were grown in LB. Samples were taken during post‐exponential growth (pe) or overnight (on), and PdeC variants expressed were visualized by immunoblotting. The dsbA mutant was included to show the position of the 33‐kD processed fragment of PdeC.
Macrocolony morphology of the degP degQ derivative of AR3110 carrying the indicated plasmids indicates high PDE activity of PdeCASS; that is, processing to the 33‐kD fragment is not required for high PDE activity of PdeCASS.

Immunoblot analysis of strain W3110 expressing the indicated PdeC variants visualized via their His6 tags.
Macrocolonies of strain AR3110 expressing the same PdeC variants as in (A) grown on salt‐free LB agar plates supplemented with Congo red (CR) for 5 days at 28°C.
Protein–protein interactions of the indicated PdeC variants were tested using a bacterial two‐hybrid (2H) system that is based on the reconstitution of adenylate cyclase (Karimova et al, 1998) which allows the utilization of maltose as a C‐source in a cya strain of Escherichia coli (resulting in red color on MacConkey/maltose agar plates). As a positive control, the leucine zipper part (‘zip’) of the yeast GCN4 protein was used. For a similar two‐hybrid analysis with corresponding PdeC variants, in which TM2 was replaced by the second transmembrane region of LacY (TM2*), see Appendix Fig S9.
To assess for dimerization in the absence of proteolytic processing of PdeCASS, an otherwise isogenic degP degQ cya recipient strain was used for the two‐hybrid analysis.
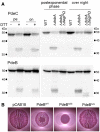
Derivatives of strain W3110 in which the chromosomal pdeC and pdeB genes carry 3′‐inserts encoding Flag tags were grown in LB medium. DTT treatment of the cultures (left panels) was as described in the legend to Fig 2. Strains shown in the right panels also carried the indicated mutations. Samples were taken during post‐exponential phase (at an OD578 of 3–3.5) and from overnight cultures and subject to immunoblot analysis with Flag tag antibodies.
Macrocolonies of Escherichia coli K‐12 strain AR3110 expressing the indicated PdeB variants under control of the tac promoter from the low copy number plasmid pCAB18 were grown on salt‐free LB (LBnoS) with the matrix dye Congo red (CR) for 5 days at 28°C.
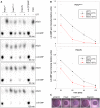
The detergent‐solubilized and purified PdeC variants as indicated were reconstituted into nanodiscs and assayed for PDE activity in the absence or presence of 6.5 mM DTT. Samples were taken at the times indicated (from top to bottom) and the remaining radiolabeled substrate c‐di‐GMP and the enzymatic product 5′‐pGpG were detected by thin‐layer chromatography and autoradiography. The purified soluble and highly active Escherichia coli PDE PdeH was used to generate radiolabeled 5′‐pGpG as a reference. The experiment was performed three times independently, with a representative example shown here.
Quantification of radiolabeled c‐di‐GMP spots as visualized in (A) was performed for the three independent experiments (open symbols for single data points) and averaged (closed symbols) to show enzymatic turnover of c‐di‐GMP by purified and nanodisc‐reconstituted PdeCEAL∆C and PdeC/N in the absence (black) and presence of DTT (red symbols).
Macrocolonies of strain AR3110 expressing the indicated PdeC variants from the low copy number plasmid pCAB18 were grown on salt‐free LB with the matrix dye Congo red (CR) for 5 days at 28°C. PdeC/N activity could be detected only upon induction with 0.1 mM IPTG (ind.).
Strain AR3110 and its derivatives carrying the indicated chromosomal mutations were grown in LB medium. Samples were taken during the growth of the cultures (numbers refer to hours of growth; on, overnight). Cellular CsgD levels were determined by immunoblotting.
Macrocolonies of strain AR3110 and its chromosomal pdeC ASS derivative were grown on salt‐free LB (LBnoS) with the matrix dye Congo red (CR) at 28°C for 2 and 5 days.
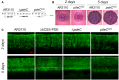
Fluorescence micrographs of vertical cryosections through the central flat regions of macrocolony biofilms of strain AR3110 and the indicated mutant derivatives were obtained after growth on salt‐free LB for 3 and 5 days at 28°C. Agar plates contained the fluorescent matrix dye thioflavin S which allows to visualize details of the extracellular matrix architecture present in the upper 45–50 μm of the macrocolonies (the entire height of Escherichia coli macrocolonies is approximately 65 μm; cells in the lower layer do not produce matrix but a mesh of entangled flagella; Serra et al, 2013a). Here, only fluorescent images are shown, the corresponding phase‐contrast and merged fluorescent/phase‐contrast images are presented in Appendix Fig S11.
References
-
- Alba BM, Gross CA (2004) Regulation of the Escherichia coli sigmaE‐dependent envelope stress response. Mol Microbiol 52: 613–619 - PubMed
-
- Almagro‐Moreno S, Root MZ, Taylor RK (2015) Role of ToxS in the proteolytic cascade of virulence regulator ToxR in Vibrio cholerae . Mol Microbiol 98: 963–976 - PubMed
-
- Barends TR, Hartmann E, Griese JJ, Beitlich T, Kirienko NV, Ryjenkov DA, Reinstein J, Shoeman RL, Gomelsky M, Schlichting I (2009) Structure and mechanism of a bacterial light‐regulated cyclic nucleotide phosphodiesterase. Nature 18: 1015–1018 - PubMed
-
- Bayburt TH, Grinkova YV, Sligar SG (2002) Self‐assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett 2: 853–856
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

