Calcium-induced release of calcium in muscle: 50 years of work and the emerging consensus
- PMID: 29514865
- PMCID: PMC5881447
- DOI: 10.1085/jgp.201711959
Calcium-induced release of calcium in muscle: 50 years of work and the emerging consensus
Abstract
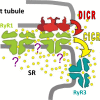
Ryanodine-sensitive intracellular Ca2+ channels (RyRs) open upon binding Ca2+ at cytosolic-facing sites. This results in concerted, self-reinforcing opening of RyRs clustered in specialized regions on the membranes of Ca2+ storage organelles (endoplasmic reticulum and sarcoplasmic reticulum), a process that produces Ca2+-induced Ca2+ release (CICR). The process is optimized to achieve large but brief and localized increases in cytosolic Ca2+ concentration, a feature now believed to be critical for encoding the multiplicity of signals conveyed by this ion. In this paper, I trace the path of research that led to a consensus on the physiological significance of CICR in skeletal muscle, beginning with its discovery. I focus on the approaches that were developed to quantify the contribution of CICR to the Ca2+ increase that results in contraction, as opposed to the flux activated directly by membrane depolarization (depolarization-induced Ca2+ release [DICR]). Although the emerging consensus is that CICR plays an important role alongside DICR in most taxa, its contribution in most mammalian muscles appears to be limited to embryogenesis. Finally, I survey the relevance of CICR, confirmed or plausible, to pathogenesis as well as the multiple questions about activation of release channels that remain unanswered after 50 years.
© 2018 Ríos.
Figures




References
-
- Airey J.A., Beck C.F., Murakami K., Tanksley S.J., Deerinck T.J., Ellisman M.H., and Sutko J.L.. 1990. Identification and localization of two triad junctional foot protein isoforms in mature avian fast twitch skeletal muscle. J. Biol. Chem. 265:14187–14194. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

