Role of Microvesicles in the Spread of Herpes Simplex Virus 1 in Oligodendrocytic Cells
- PMID: 29514899
- PMCID: PMC5923088
- DOI: 10.1128/JVI.00088-18
Role of Microvesicles in the Spread of Herpes Simplex Virus 1 in Oligodendrocytic Cells
Abstract
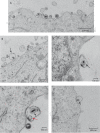
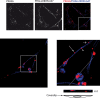
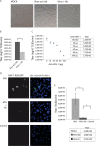
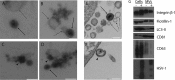
Herpes simplex virus 1 (HSV-1) is a neurotropic pathogen that can infect many types of cells and establishes latent infections in the neurons of sensory ganglia. In some cases, the virus spreads into the central nervous system, causing encephalitis or meningitis. Cells infected with several different types of viruses may secrete microvesicles (MVs) containing viral proteins and RNAs. In some instances, extracellular microvesicles harboring infectious virus have been found. Here we describe the features of shedding microvesicles released by the human oligodendroglial HOG cell line infected with HSV-1 and their participation in the viral cycle. Using transmission electron microscopy, we detected for the first time microvesicles containing HSV-1 virions. Interestingly, the Chinese hamster ovary (CHO) cell line, which is resistant to infection by free HSV-1 virions, was susceptible to HSV-1 infection after being exposed to virus-containing microvesicles. Therefore, our results indicate for the first time that MVs released by infected cells contain virions, are endocytosed by naive cells, and lead to a productive infection. Furthermore, infection of CHO cells was not completely neutralized when virus-containing microvesicles were preincubated with neutralizing anti-HSV-1 antibodies. The lack of complete neutralization and the ability of MVs to infect nectin-1/HVEM-negative CHO-K1 cells suggest a novel way for HSV-1 to spread to and enter target cells. Taken together, our results suggest that HSV-1 could spread through microvesicles to expand its tropism and that microvesicles could shield the virus from neutralizing antibodies as a possible mechanism to escape the host immune response.IMPORTANCE Herpes simplex virus 1 (HSV-1) is a neurotropic pathogen that can infect many types of cells and establishes latent infections in neurons. Extracellular vesicles are a heterogeneous group of membrane vesicles secreted by most cell types. Microvesicles, which are extracellular vesicles which derive from the shedding of the plasma membrane, isolated from the supernatant of HSV-1-infected HOG cells were analyzed to find out whether they were involved in the viral cycle. The importance of our investigation lies in the detection, for the first time, of microvesicles containing HSV-1 virions. In addition, virus-containing microvesicles were endocytosed into CHO-K1 cells and were able to actively infect these otherwise nonpermissive cells. Finally, the infection of CHO cells with these virus-containing microvesicles was not completely neutralized by anti-HSV-1 antibodies, suggesting that these extracellular vesicles might shield the virus from neutralizing antibodies as a possible mechanism of immune evasion.
Keywords: extracellular vesicles; herpes simplex virus; microvesicles; oligodendrocytes; viral spread.
Copyright © 2018 Bello-Morales et al.
Figures


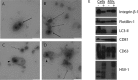
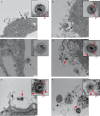
References
-
- Wald A, Corey L. 2007. Persistence in the population: epidemiology, transmission. In Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, United Kingdom. - PubMed
-
- Bernstein DI, Bellamy AR, Hook EW III, Levin MJ, Wald A, Ewell MG, Wolff PA, Deal CD, Heineman TC, Dubin G, Belshe RB. 2013. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis 56:344–351. doi:10.1093/cid/cis891. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

