Human T Lymphocytes Are Permissive for Dengue Virus Replication
- PMID: 29514900
- PMCID: PMC5923084
- DOI: 10.1128/JVI.02181-17
Human T Lymphocytes Are Permissive for Dengue Virus Replication
Abstract
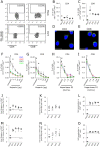
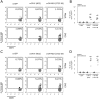
Dengue virus (DV) infection can cause either a self-limiting flu-like disease or a threatening hemorrhage that may evolve to shock and death. A variety of cell types, such as dendritic cells, monocytes, and B cells, can be infected by DV. However, despite the role of T lymphocytes in the control of DV replication, there remains a paucity of information on possible DV-T cell interactions during the disease course. In the present study, we have demonstrated that primary human naive CD4+ and CD8+ T cells are permissive for DV infection. Importantly, both T cell subtypes support viral replication and secrete viable virus particles. DV infection triggers the activation of both CD4+ and CD8+ T lymphocytes, but preactivation of T cells reduces the susceptibility of T cells to DV infection. Interestingly, the cytotoxicity-inducing protein granzyme A is highly secreted by human CD4+ but not CD8+ T cells after exposure to DV in vitro Additionally, using annexin V and polycaspase assays, we have demonstrated that T lymphocytes, in contrast to monocytes, are resistant to DV-induced apoptosis. Strikingly, both CD4+ and CD8+ T cells were found to be infected with DV in acutely infected dengue patients. Together, these results show that T cells are permissive for DV infection in vitro and in vivo, suggesting that this cell population may be a viral reservoir during the acute phase of the disease.IMPORTANCE Infection by dengue virus (DV) causes a flu-like disease that can evolve to severe hemorrhaging and death. T lymphocytes are important cells that regulate antibody secretion by B cells and trigger the death of infected cells. However, little is known about the direct interaction between DV and T lymphocytes. Here, we show that T lymphocytes from healthy donors are susceptible to infection by DV, leading to cell activation. Additionally, T cells seem to be resistant to DV-induced apoptosis, suggesting a potential role as a viral reservoir in humans. Finally, we show that both CD4+ and CD8+ T lymphocytes from acutely infected DV patients are infected by DV. Our results raise new questions about DV pathogenesis and vaccine development.
Keywords: T lymphocytes; dengue virus; replication.
Copyright © 2018 American Society for Microbiology.
Figures

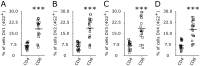






Similar articles
-
Human CD4+ T Cell Responses to an Attenuated Tetravalent Dengue Vaccine Parallel Those Induced by Natural Infection in Magnitude, HLA Restriction, and Antigen Specificity.J Virol. 2017 Feb 14;91(5):e02147-16. doi: 10.1128/JVI.02147-16. Print 2017 Mar 1. J Virol. 2017. PMID: 27974563 Free PMC article.
-
Dengue virus-specific CD4+ and CD8+ T lymphocytes target NS1, NS3 and NS5 in infected Indian rhesus macaques.Immunogenetics. 2012 Feb;64(2):111-21. doi: 10.1007/s00251-011-0566-0. Epub 2011 Sep 1. Immunogenetics. 2012. PMID: 21881953
-
Virus-specific T lymphocytes home to the skin during natural dengue infection.Sci Transl Med. 2015 Mar 11;7(278):278ra35. doi: 10.1126/scitranslmed.aaa0526. Sci Transl Med. 2015. PMID: 25761891
-
Understanding the Human T Cell Response to Dengue Virus.Adv Exp Med Biol. 2018;1062:241-250. doi: 10.1007/978-981-10-8727-1_17. Adv Exp Med Biol. 2018. PMID: 29845537 Review.
-
Human T Cell Response to Dengue Virus Infection.Front Immunol. 2019 Sep 4;10:2125. doi: 10.3389/fimmu.2019.02125. eCollection 2019. Front Immunol. 2019. PMID: 31552052 Free PMC article. Review.
Cited by
-
Immunologic Crosstalk and Host-Specific Immune Signature Associated with Dengue.ACS Omega. 2024 Aug 20;9(36):37418-37429. doi: 10.1021/acsomega.4c02506. eCollection 2024 Sep 10. ACS Omega. 2024. PMID: 39281909 Free PMC article. Review.
-
Addition of Partial Envelope Domain II into Envelope Domain III of Dengue Virus Antigen Potentiates the Induction of Virus-Neutralizing Antibodies and Induces Protective Immunity.Vaccines (Basel). 2020 Feb 15;8(1):88. doi: 10.3390/vaccines8010088. Vaccines (Basel). 2020. PMID: 32075300 Free PMC article.
-
Dengue virus co-opts innate type 2 pathways to escape early control of viral replication.Commun Biol. 2022 Jul 22;5(1):735. doi: 10.1038/s42003-022-03682-5. Commun Biol. 2022. PMID: 35869167 Free PMC article.
-
Hijacking the Host Immune Cells by Dengue Virus: Molecular Interplay of Receptors and Dengue Virus Envelope.Microorganisms. 2019 Sep 6;7(9):323. doi: 10.3390/microorganisms7090323. Microorganisms. 2019. PMID: 31489877 Free PMC article. Review.
-
Dengue Infection Complicated by Hemophagocytic Lymphohistiocytosis: Experiences From 180 Patients With Severe Dengue.Clin Infect Dis. 2020 May 23;70(11):2247-2255. doi: 10.1093/cid/ciz499. Clin Infect Dis. 2020. PMID: 31188423 Free PMC article.
References
-
- WHO. 2009. Dengue guidelines for diagnosis, treatment, prevention and control. WHO, Geneva, Switzerland. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

