Reovirus-Induced Apoptosis in the Intestine Limits Establishment of Enteric Infection
- PMID: 29514905
- PMCID: PMC5923068
- DOI: 10.1128/JVI.02062-17
Reovirus-Induced Apoptosis in the Intestine Limits Establishment of Enteric Infection
Abstract
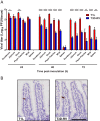
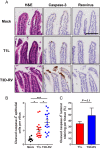
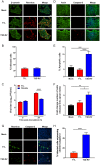
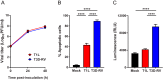
Several viruses induce intestinal epithelial cell death during enteric infection. However, it is unclear whether proapoptotic capacity promotes or inhibits replication in this tissue. We infected mice with two reovirus strains that infect the intestine but differ in the capacity to alter immunological tolerance to new food antigen. Infection with reovirus strain T1L, which induces an inflammatory immune response to fed antigen, is prolonged in the intestine, whereas T3D-RV, which does not induce this response, is rapidly cleared from the intestine. Compared with T1L, T3D-RV infection triggered apoptosis of intestinal epithelial cells and subsequent sloughing of dead cells into the intestinal lumen. We conclude that the infection advantage of T1L derives from its capacity to subvert host restriction by epithelial cell apoptosis, providing a possible mechanism by which T1L enhances inflammatory signals during antigen feeding. Using a panel of T1L × T3D-RV reassortant viruses, we identified the viral M1 and M2 gene segments as determinants of reovirus-induced apoptosis in the intestine. Expression of the T1L M1 and M2 genes in a T3D-RV background was sufficient to limit epithelial cell apoptosis and enhance viral infection to levels displayed by T1L. These findings define additional reovirus gene segments required for enteric infection of mice and illuminate the antiviral effect of intestinal epithelial cell apoptosis in limiting enteric viral infection. Viral strain-specific differences in the capacity to infect the intestine may be useful in identifying viruses capable of ameliorating tolerance to fed antigen in autoimmune conditions like celiac disease.IMPORTANCE Acute viral infections are thought to be cleared by the host with few lasting consequences. However, there may be much broader and long-lasting effects of viruses on immune homeostasis. Infection with reovirus, a common, nonpathogenic virus, triggers inflammation against innocuous food antigens, implicating this virus in the development of celiac disease, an autoimmune intestinal disorder triggered by exposure to dietary gluten. Using two reovirus strains that differ in the capacity to abrogate oral tolerance, we found that strain-specific differences in the capacity to replicate in the intestine inversely correlate with the capacity to induce apoptotic death of intestinal epithelial cells, providing a host-mediated process to restrict intestinal infection. This work contributes new knowledge about virus-host interactions in the intestine and establishes a foundation for future studies to define mechanisms by which viruses break oral tolerance in celiac disease.
Keywords: apoptosis; cell death; enteroid; gastrointestinal infection; mucosal immunity; reovirus.
Copyright © 2018 American Society for Microbiology.
Figures
Similar articles
-
Strain-specific differences in reovirus infection of murine macrophages segregate with polymorphisms in viral outer-capsid protein σ3.J Virol. 2024 Nov 19;98(11):e0114724. doi: 10.1128/jvi.01147-24. Epub 2024 Oct 21. J Virol. 2024. PMID: 39431846 Free PMC article.
-
The M2 Gene Is a Determinant of Reovirus-Induced Myocarditis.J Virol. 2022 Jan 26;96(2):e0187921. doi: 10.1128/JVI.01879-21. Epub 2021 Nov 10. J Virol. 2022. PMID: 34757847 Free PMC article.
-
Expression of Ifnlr1 on Intestinal Epithelial Cells Is Critical to the Antiviral Effects of Interferon Lambda against Norovirus and Reovirus.J Virol. 2017 Mar 13;91(7):e02079-16. doi: 10.1128/JVI.02079-16. Print 2017 Apr 1. J Virol. 2017. PMID: 28077655 Free PMC article.
-
Mechanisms of apoptosis during reovirus infection.Curr Top Microbiol Immunol. 2005;289:1-24. doi: 10.1007/3-540-27320-4_1. Curr Top Microbiol Immunol. 2005. PMID: 15791949 Free PMC article. Review.
-
Mechanisms of reovirus bloodstream dissemination.Adv Virus Res. 2013;87:1-35. doi: 10.1016/B978-0-12-407698-3.00001-6. Adv Virus Res. 2013. PMID: 23809919 Free PMC article. Review.
Cited by
-
Reovirus Efficiently Reassorts Genome Segments during Coinfection and Superinfection.J Virol. 2022 Sep 28;96(18):e0091022. doi: 10.1128/jvi.00910-22. Epub 2022 Sep 12. J Virol. 2022. PMID: 36094315 Free PMC article.
-
Mechanosensitive extrusion of Enterovirus A71-infected cells from colonic organoids.Nat Microbiol. 2023 Apr;8(4):629-639. doi: 10.1038/s41564-023-01339-5. Epub 2023 Mar 13. Nat Microbiol. 2023. PMID: 36914754 Free PMC article.
-
Inhibition of mycobacteria proliferation in macrophages by low cisplatin concentration through phosphorylated p53-related apoptosis pathway.PLoS One. 2023 Jan 31;18(1):e0281170. doi: 10.1371/journal.pone.0281170. eCollection 2023. PLoS One. 2023. PMID: 36719870 Free PMC article.
-
Using Diverse Model Systems to Define Intestinal Epithelial Defenses to Enteric Viral Infections.Cell Host Microbe. 2020 Mar 11;27(3):329-344. doi: 10.1016/j.chom.2020.02.003. Cell Host Microbe. 2020. PMID: 32164844 Free PMC article. Review.
-
Lymph node sharing between pancreas, gut, and liver leads to immune crosstalk and regulation of pancreatic autoimmunity.Immunity. 2023 Sep 12;56(9):2070-2085.e11. doi: 10.1016/j.immuni.2023.07.008. Epub 2023 Aug 8. Immunity. 2023. PMID: 37557168 Free PMC article.
References
-
- Bouziat R, Hinterleitner R, Brown JJ, Stencel-Baerenwald JE, Ikizler M, Mayassi T, Meisel M, Kim SM, Discepolo V, Pruijssers AJ, Ernest JD, Iskarpatyoti JA, Costes LM, Lawrence I, Palanski BA, Varma M, Zurenski MA, Khomandiak S, McAllister N, Aravamudhan P, Boehme KW, Hu F, Samsom JN, Reinecker HC, Kupfer SS, Guandalini S, Semrad CE, Abadie V, Khosla C, Barreiro LB, Xavier RJ, Ng A, Dermody TS, Jabri B. 2017. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 356:44–50. doi:10.1126/science.aah5298. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

