Distinct Immunogenicity and Efficacy of Poxvirus-Based Vaccine Candidates against Ebola Virus Expressing GP and VP40 Proteins
- PMID: 29514907
- PMCID: PMC5952144
- DOI: 10.1128/JVI.00363-18
Distinct Immunogenicity and Efficacy of Poxvirus-Based Vaccine Candidates against Ebola Virus Expressing GP and VP40 Proteins
Abstract
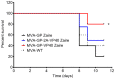
Zaire and Sudan ebolavirus species cause a severe disease in humans and nonhuman primates (NHPs) characterized by a high mortality rate. There are no licensed therapies or vaccines against Ebola virus disease (EVD), and the recent 2013 to 2016 outbreak in West Africa highlighted the need for EVD-specific medical countermeasures. Here, we generated and characterized head-to-head the immunogenicity and efficacy of five vaccine candidates against Zaire ebolavirus (EBOV) and Sudan ebolavirus (SUDV) based on the highly attenuated poxvirus vector modified vaccinia virus Ankara (MVA) expressing either the virus glycoprotein (GP) or GP together with the virus protein 40 (VP40) forming virus-like particles (VLPs). In a human monocytic cell line, the different MVA vectors (termed MVA-EBOVs and MVA-SUDVs) triggered robust innate immune responses, with production of beta interferon (IFN-β), proinflammatory cytokines, and chemokines. Additionally, several innate immune cells, such as dendritic cells, neutrophils, and natural killer cells, were differentially recruited in the peritoneal cavity of mice inoculated with MVA-EBOVs. After immunization of mice with a homologous prime/boost protocol (MVA/MVA), total IgG antibodies against GP or VP40 from Zaire and Sudan ebolavirus were differentially induced by these vectors, which were mainly of the IgG1 and IgG3 isotypes. Remarkably, an MVA-EBOV construct coexpressing GP and VP40 protected chimeric mice challenged with EBOV to a greater extent than a vector expressing GP alone. These results support the consideration of MVA-EBOVs and MVA-SUDVs expressing GP and VP40 and producing VLPs as best-in-class potential vaccine candidates against EBOV and SUDV.IMPORTANCE EBOV and SUDV cause a severe hemorrhagic fever affecting humans and NHPs. Since their discovery in 1976, they have caused several sporadic epidemics, with the recent outbreak in West Africa from 2013 to 2016 being the largest and most severe, with more than 11,000 deaths being reported. Although some vaccines are in advanced clinical phases, less expensive, safer, and more effective licensed vaccines are desirable. We generated and characterized head-to-head the immunogenicity and efficacy of five novel vaccines against EBOV and SUDV based on the poxvirus MVA expressing GP or GP and VP40. The expression of GP and VP40 leads to the formation of VLPs. These MVA-EBOV and MVA-SUDV recombinants triggered robust innate and humoral immune responses in mice. Furthermore, MVA-EBOV recombinants expressing GP and VP40 induced high protection against EBOV in a mouse challenge model. Thus, MVA expressing GP and VP40 and producing VLPs is a promising vaccine candidate against EBOV and SUDV.
Keywords: Ebola; GP; MVA; VP40; immunogenicity; mice; poxvirus; protection.
Copyright © 2018 American Society for Microbiology.
Figures



Similar articles
-
Recombinant Modified Vaccinia Virus Ankara Generating Ebola Virus-Like Particles.J Virol. 2017 May 12;91(11):e00343-17. doi: 10.1128/JVI.00343-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28331098 Free PMC article.
-
A Chimeric Sudan Virus-Like Particle Vaccine Candidate Produced by a Recombinant Baculovirus System Induces Specific Immune Responses in Mice and Horses.Viruses. 2020 Jan 3;12(1):64. doi: 10.3390/v12010064. Viruses. 2020. PMID: 31947873 Free PMC article.
-
A Bivalent, Spherical Virus-Like Particle Vaccine Enhances Breadth of Immune Responses against Pathogenic Ebola Viruses in Rhesus Macaques.J Virol. 2020 Apr 16;94(9):e01884-19. doi: 10.1128/JVI.01884-19. Print 2020 Apr 16. J Virol. 2020. PMID: 32075939 Free PMC article.
-
Correlates of vaccine-induced protective immunity against Ebola virus disease.Semin Immunol. 2018 Oct;39:65-72. doi: 10.1016/j.smim.2018.07.003. Epub 2018 Jul 21. Semin Immunol. 2018. PMID: 30041831 Review.
-
Ebola vaccine trials: progress in vaccine safety and immunogenicity.Expert Rev Vaccines. 2019 Dec;18(12):1229-1242. doi: 10.1080/14760584.2019.1698952. Expert Rev Vaccines. 2019. PMID: 31779496 Review.
Cited by
-
A Single Dose of Modified Vaccinia Ankara Expressing Lassa Virus-like Particles Protects Mice from Lethal Intra-cerebral Virus Challenge.Pathogens. 2019 Aug 28;8(3):133. doi: 10.3390/pathogens8030133. Pathogens. 2019. PMID: 31466243 Free PMC article.
-
COVID-19 vaccine candidates based on modified vaccinia virus Ankara expressing the SARS-CoV-2 spike induce robust T- and B-cell immune responses and full efficacy in mice.J Virol. 2021 Mar 10;95(7):e02260-20. doi: 10.1128/JVI.02260-20. Epub 2021 Jan 7. J Virol. 2021. PMID: 33414159 Free PMC article.
-
A Vaccine Based on a Modified Vaccinia Virus Ankara Vector Expressing Zika Virus Structural Proteins Controls Zika Virus Replication in Mice.Sci Rep. 2018 Nov 26;8(1):17385. doi: 10.1038/s41598-018-35724-6. Sci Rep. 2018. PMID: 30478418 Free PMC article.
-
DNA-launched RNA replicon vaccines induce potent anti-Ebolavirus immune responses that can be further improved by a recombinant MVA boost.Sci Rep. 2018 Aug 20;8(1):12459. doi: 10.1038/s41598-018-31003-6. Sci Rep. 2018. PMID: 30127450 Free PMC article.
-
The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies.Front Immunol. 2020 Jul 21;11:1817. doi: 10.3389/fimmu.2020.01817. eCollection 2020. Front Immunol. 2020. PMID: 32793245 Free PMC article. Review.
References
-
- Bukreyev AA, Chandran K, Dolnik O, Dye JM, Ebihara H, Leroy EM, Muhlberger E, Netesov SV, Patterson JL, Paweska JT, Saphire EO, Smither SJ, Takada A, Towner JS, Volchkov VE, Warren TK, Kuhn JH. 2014. Discussions and decisions of the 2012-2014 International Committee on Taxonomy of Viruses (ICTV) Filoviridae Study Group, January 2012-June 2013. Arch Virol 159:821–830. doi:10.1007/s00705-013-1846-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

