Porcine MKRN1 Modulates the Replication and Pathogenesis of Porcine Circovirus Type 2 by Inducing Capsid Protein Ubiquitination and Degradation
- PMID: 29514908
- PMCID: PMC5952126
- DOI: 10.1128/JVI.00100-18
Porcine MKRN1 Modulates the Replication and Pathogenesis of Porcine Circovirus Type 2 by Inducing Capsid Protein Ubiquitination and Degradation
Erratum in
-
Correction for Wang et al., "Porcine MKRN1 Modulates the Replication and Pathogenesis of Porcine Circovirus Type 2 by Inducing Capsid Protein Ubiquitination and Degradation".J Virol. 2018 Oct 12;92(21):e01351-18. doi: 10.1128/JVI.01351-18. Print 2018 Nov 1. J Virol. 2018. PMID: 30315091 Free PMC article. No abstract available.
Abstract
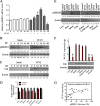
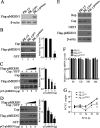
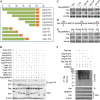
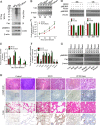
Porcine circovirus type 2 (PCV2) capsid protein (Cap) is a unique structure protein that plays pivotal roles in the process of viral replication and pathogenesis. Herein, we characterized a putative porcine Makorin RING finger protein 1 (pMKRN1) variant, an N-terminal-truncated variant of putative full-size porcine MKRN1 which has a unique expression pattern resulting from the porcine mkrn1 gene and which interacts with PCV2 Cap. A domain mapping assay showed that the C terminus of pMKRN1 and fragments (amino acids 108 to 198) of Cap are required for this interaction. PCV2 transiently upregulated pMKRN1 in PK-15 cells, but persistent viral infection downregulated pMKRN1 in major pathological tissues of PCV2-infected piglets. Overexpression of pMKRN1 significantly inhibited the generation of progeny PCV2 via ubiquitination and degradation of Cap, whereas knockout of pMKRN1 blocked Cap degradation and promoted progeny virus replication. pMKRN1 specifically targeted PCV2 Cap lysine residues 164, 179, and 191 to induce polyubiquitination and subsequent degradation. Mutation of either of the three lysine residues in the Cap protein or mutation of the histidine at residue 243 within the RING finger domain of pMKRN1 abrogated the E3 ligase activity of pMKRN1, rendering cells incapable of inducing Cap ubiquitination and degradation. Consistent with this finding, a Cap ubiquitination-deficient PCV2 strain showed enhanced virus replication and produced severe histological lesions in the lung and lymph node tissues compared with wild-type PCV2. Taken together, the results presented here suggest that PCV2 downregulates the pMKRN1 variant to avoid pMKRN1-mediated Cap ubiquitination and degradation, thus promoting viral replication and pathogenesis in its targeted tissues.IMPORTANCE Porcine circovirus type 2 is the pathogen to which pigs are the most susceptible, causing immense economic losses in the global swine industry, but whether host cells have developed some strategies to prevent viral replication is still unclear. Here, we found that porcine MKRN1 (pMKRN1) was upregulated in the early stage of PCV2 infection and mediated the polyubiquitination and degradation of Cap protein to block PCV2 replication, yet persistent PCV2 infection downregulated pMKRN1 levels to avoid degradation, promoting viral replication and pathogenesis in its targeted tissues. These data present new insight into the molecular mechanisms underlying the antiviral effects of pMKRN1 E3 ligase during PCV2 infection and also suggest potential new control measures for PCV2 outbreaks.
Keywords: PCV2; pMKRN1; replication; ubiquitination.
Copyright © 2018 American Society for Microbiology.
Figures




Similar articles
-
E3 ligase RNF2 inhibits porcine circovirus type 3 replication by targeting its capsid protein for ubiquitination-dependent degradation.J Virol. 2024 Aug 20;98(8):e0022324. doi: 10.1128/jvi.00223-24. Epub 2024 Jul 24. J Virol. 2024. PMID: 39046246 Free PMC article.
-
Cellular p32 Is a Critical Regulator of Porcine Circovirus Type 2 Nuclear Egress.J Virol. 2019 Nov 13;93(23):e00979-19. doi: 10.1128/JVI.00979-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31511386 Free PMC article.
-
Ubiquitination-dependent degradation of DHX36 mediated by porcine circovirus type 3 capsid protein.Virology. 2025 Mar;604:110419. doi: 10.1016/j.virol.2025.110419. Epub 2025 Jan 21. Virology. 2025. PMID: 39862752
-
Porcine circovirus type 2 (PCV2): pathogenesis and interaction with the immune system.Annu Rev Anim Biosci. 2013 Jan;1:43-64. doi: 10.1146/annurev-animal-031412-103720. Epub 2013 Jan 3. Annu Rev Anim Biosci. 2013. PMID: 25387012 Review.
-
Concurrent infections are important for expression of porcine circovirus associated disease.Virus Res. 2012 Mar;164(1-2):20-32. doi: 10.1016/j.virusres.2011.09.014. Epub 2011 Sep 16. Virus Res. 2012. PMID: 21959087 Free PMC article. Review.
Cited by
-
Porcine DNAJB6 promotes PCV2 replication via enhancing the formation of autophagy in host cells.Vet Res. 2020 May 7;51(1):61. doi: 10.1186/s13567-020-00783-z. Vet Res. 2020. PMID: 32381067 Free PMC article.
-
Heme oxygenase-1 is an equid alphaherpesvirus 8 replication restriction host protein and suppresses viral replication via the PKCβ/ERK1/ERK2 and NO/cGMP/PKG pathway.Microbiol Spectr. 2024 Apr 2;12(4):e0322023. doi: 10.1128/spectrum.03220-23. Epub 2024 Mar 5. Microbiol Spectr. 2024. PMID: 38441979 Free PMC article.
-
Characterization of a duck circovirus propagated in duck embryos: from genome to pathogenesis in SPF ducks.Poult Sci. 2025 Jun 11;104(9):105431. doi: 10.1016/j.psj.2025.105431. Online ahead of print. Poult Sci. 2025. PMID: 40527129 Free PMC article.
-
Blebbistatin as a novel antiviral agent targeting equid herpesvirus type 8.Front Vet Sci. 2024 Jun 5;11:1390304. doi: 10.3389/fvets.2024.1390304. eCollection 2024. Front Vet Sci. 2024. PMID: 38898998 Free PMC article.
-
PoRVA G9P[23] and G5P[7] infections differentially promote PEDV replication by reprogramming glutamine metabolism.PLoS Pathog. 2024 Jun 21;20(6):e1012305. doi: 10.1371/journal.ppat.1012305. eCollection 2024 Jun. PLoS Pathog. 2024. PMID: 38905309 Free PMC article.
References
-
- Thomson J, Smith B, Allan G, McNeilly F, McVicar C. 2000. PDNS, PMWS and porcine circovirus type 2 in Scotland. Porcine dermatitis and nephropathy syndrome. Post-weaning multisystemic wasting syndrome. Vet Rec 146:651–652. - PubMed
-
- Pineyro PE, Kenney SP, Gimenez-Lirola LG, Opriessnig T, Tian D, Heffron CL, Meng XJ. 2016. Evaluation of the use of non-pathogenic porcine circovirus type 1 as a vaccine delivery virus vector to express antigenic epitopes of porcine reproductive and respiratory syndrome virus. Virus Res 213:100–108. doi: 10.1016/j.virusres.2015.11.005. - DOI - PubMed
-
- Mankertz A, Mankertz J, Wolf K, Buhk HJ. 1998. Identification of a protein essential for replication of porcine circovirus. J Gen Virol 79(Pt 2):381–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

