CDK2 inhibitors as candidate therapeutics for cisplatin- and noise-induced hearing loss
- PMID: 29514916
- PMCID: PMC5881471
- DOI: 10.1084/jem.20172246
CDK2 inhibitors as candidate therapeutics for cisplatin- and noise-induced hearing loss
Abstract
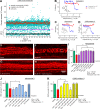
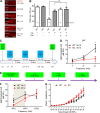
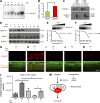
Hearing loss caused by aging, noise, cisplatin toxicity, or other insults affects 360 million people worldwide, but there are no Food and Drug Administration-approved drugs to prevent or treat it. We screened 4,385 small molecules in a cochlear cell line and identified 10 compounds that protected against cisplatin toxicity in mouse cochlear explants. Among them, kenpaullone, an inhibitor of multiple kinases, including cyclin-dependent kinase 2 (CDK2), protected zebrafish lateral-line neuromasts from cisplatin toxicity and, when delivered locally, protected adult mice and rats against cisplatin- and noise-induced hearing loss. CDK2-deficient mice displayed enhanced resistance to cisplatin toxicity in cochlear explants and to cisplatin- and noise-induced hearing loss in vivo. Mechanistically, we showed that kenpaullone directly inhibits CDK2 kinase activity and reduces cisplatin-induced mitochondrial production of reactive oxygen species, thereby enhancing cell survival. Our experiments have revealed the proapoptotic function of CDK2 in postmitotic cochlear cells and have identified promising therapeutics for preventing hearing loss.
© 2018 Teitz et al.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

