The yeast arrestin-related protein Bul1 is a novel actor of glucose-induced endocytosis
- PMID: 29514933
- PMCID: PMC5921569
- DOI: 10.1091/mbc.E17-07-0466
The yeast arrestin-related protein Bul1 is a novel actor of glucose-induced endocytosis
Abstract
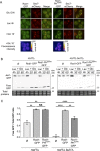
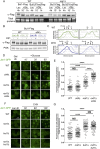
Yeast cells have a remarkable ability to adapt to nutritional changes in their environment. During adaptation, nutrient-signaling pathways drive the selective endocytosis of nutrient transporters present at the cell surface. A current challenge is to understand the mechanistic basis of this regulation. Transporter endocytosis is triggered by their ubiquitylation, which involves the ubiquitin ligase Rsp5 and its adaptors of the arrestin-related family (ART). This step is highly regulated by nutrient availability. For instance, the monocarboxylate transporter Jen1 is ubiquitylated, endocytosed, and degraded upon exposure to glucose. The ART protein Rod1 is required for this overall process; yet Rod1 rather controls Jen1 trafficking later in the endocytic pathway and is almost dispensable for Jen1 internalization. Thus, how glucose triggers Jen1 internalization remains unclear. We report that another ART named Bul1, but not its paralogue Bul2, contributes to Jen1 internalization. Bul1 responds to glucose availability, and preferentially acts at the plasma membrane for Jen1 internalization. Thus, multiple ARTs can act sequentially along the endocytic pathway to control transporter homeostasis. Moreover, Bul1 is in charge of Jen1 endocytosis after cycloheximide treatment, suggesting that the functional redundancy of ARTs may be explained by their ability to interact with multiple cargoes in various conditions.
Figures



Similar articles
-
The C-terminal region of the yeast monocarboxylate transporter Jen1 acts as a glucose signal-responding degron recognized by the α-arrestin Rod1.J Biol Chem. 2018 Jul 13;293(28):10926-10936. doi: 10.1074/jbc.RA117.001062. Epub 2018 May 22. J Biol Chem. 2018. PMID: 29789424 Free PMC article.
-
Integrated control of transporter endocytosis and recycling by the arrestin-related protein Rod1 and the ubiquitin ligase Rsp5.Elife. 2014 Nov 7;3:e03307. doi: 10.7554/eLife.03307. Elife. 2014. PMID: 25380227 Free PMC article.
-
Glucose-induced ubiquitylation and endocytosis of the yeast Jen1 transporter: role of lysine 63-linked ubiquitin chains.J Biol Chem. 2009 Jul 17;284(29):19228-36. doi: 10.1074/jbc.M109.008318. Epub 2009 May 11. J Biol Chem. 2009. PMID: 19433580 Free PMC article.
-
The α-arrestin family of ubiquitin ligase adaptors links metabolism with selective endocytosis.Biol Cell. 2021 Apr;113(4):183-219. doi: 10.1111/boc.202000137. Epub 2021 Feb 1. Biol Cell. 2021. PMID: 33314196 Review.
-
Ubiquitin ligase adaptors: regulators of ubiquitylation and endocytosis of plasma membrane proteins.Exp Cell Res. 2009 May 15;315(9):1574-83. doi: 10.1016/j.yexcr.2008.11.014. Epub 2008 Dec 3. Exp Cell Res. 2009. PMID: 19070615 Review.
Cited by
-
Adaptors as the regulators of HECT ubiquitin ligases.Cell Death Differ. 2021 Feb;28(2):455-472. doi: 10.1038/s41418-020-00707-6. Epub 2021 Jan 5. Cell Death Differ. 2021. PMID: 33402750 Free PMC article. Review.
-
Interaction of ARRDC4 With GLUT1 Mediates Metabolic Stress in the Ischemic Heart.Circ Res. 2022 Sep 2;131(6):510-527. doi: 10.1161/CIRCRESAHA.122.321351. Epub 2022 Aug 11. Circ Res. 2022. PMID: 35950500 Free PMC article.
-
The Bul1/2 Alpha-Arrestins Promote Ubiquitylation and Endocytosis of the Can1 Permease upon Cycloheximide-Induced TORC1-Hyperactivation.Int J Mol Sci. 2021 Sep 22;22(19):10208. doi: 10.3390/ijms221910208. Int J Mol Sci. 2021. PMID: 34638549 Free PMC article.
-
Endocytosis of nutrient transporters in fungi: The ART of connecting signaling and trafficking.Comput Struct Biotechnol J. 2021 Mar 19;19:1713-1737. doi: 10.1016/j.csbj.2021.03.013. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 33897977 Free PMC article. Review.
-
Profile of Membrane Cargo Trafficking Proteins and Transporters Expressed under N Source Derepressing Conditions in Aspergillus nidulans.J Fungi (Basel). 2021 Jul 14;7(7):560. doi: 10.3390/jof7070560. J Fungi (Basel). 2021. PMID: 34356937 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

