Structural basis for the regulatory interaction of the methylglyoxal synthase MgsA with the carbon flux regulator Crh in Bacillus subtilis
- PMID: 29514981
- PMCID: PMC5912461
- DOI: 10.1074/jbc.RA117.001289
Structural basis for the regulatory interaction of the methylglyoxal synthase MgsA with the carbon flux regulator Crh in Bacillus subtilis
Abstract
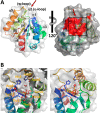
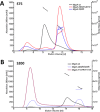
Utilization of energy-rich carbon sources such as glucose is fundamental to the evolutionary success of bacteria. Glucose can be catabolized via glycolysis for feeding the intermediary metabolism. The methylglyoxal synthase MgsA produces methylglyoxal from the glycolytic intermediate dihydroxyacetone phosphate. Methylglyoxal is toxic, requiring stringent regulation of MgsA activity. In the Gram-positive bacterium Bacillus subtilis, an interaction with the phosphoprotein Crh controls MgsA activity. In the absence of preferred carbon sources, Crh is present in the nonphosphorylated state and binds to and thereby inhibits MgsA. To better understand the mechanism of regulation of MgsA, here we performed biochemical and structural analyses of B. subtilis MgsA and of its interaction with Crh. Our results indicated that MgsA forms a hexamer (i.e. a trimer of dimers) in the crystal structure, whereas it seems to exist in an equilibrium between a dimer and hexamer in solution. In the hexamer, two alternative dimers could be distinguished, but only one appeared to prevail in solution. Further analysis strongly suggested that the hexamer is the biologically active form. In vitro cross-linking studies revealed that Crh interacts with the N-terminal helices of MgsA and that the Crh-MgsA binding inactivates MgsA by distorting and thereby blocking its active site. In summary, our results indicate that dimeric and hexameric MgsA species exist in an equilibrium in solution, that the hexameric species is the active form, and that binding to Crh deforms and blocks the active site in MgsA.
Keywords: Bacillus; Crh; HPr; bacteria; bacterial genetics; bacterial metabolism; catabolite regulation; crystal structure; glucose catabolism; glycolysis; metabolic regulation; methylglyoxal synthase; methylglyoxal toxicity; phosphoprotein; prokaryotic signal-transduction; protein cross-linking; protein structure; protein-protein interaction.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

