Celecoxib alleviates nonalcoholic fatty liver disease by restoring autophagic flux
- PMID: 29515134
- PMCID: PMC5841322
- DOI: 10.1038/s41598-018-22339-0
Celecoxib alleviates nonalcoholic fatty liver disease by restoring autophagic flux
Abstract
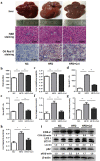
Nonalcoholic fatty liver disease (NAFLD) is a kind of liver lipid synthesis and degradation imbalance related with metabolic syndrome. Celecoxib shows the function of ameliorating NAFLD, but the underlying mechanisms remain unknown. Here, we discuss the possible mechanisms of celecoxib alleviating NAFLD by restoring autophagic flux. Lipids were accumulated in L02 cells treated with palmitate as well as SD rats fed with high-fat diet. Western blot showed that LC3 II/I was higher and p62 was lower on the early stage of steatosis while on the late stage both of them were higher, indicating that autophagic flux was activated on the early stage of steatosis, but blocked on the late stage. Rapamycin alleviated steatosis with activating autophagic flux while chloroquine aggravated steatosis with inhibiting autophagic flux. COX-2 siRNA and celecoxib were used to inhibit COX-2. Western blot and RFP-GFP-LC3 double fluorescence system indicated that celecoxib could ameliorate steatosis and restore autophagic flux in L02 cells treated with palmitate as well as SD rats fed with high-fat diet. In conclusion, celecoxib partially restores autophagic flux via downregulation of COX-2 and alleviates steatosis in vitro and in vivo.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

