Results of the national organised colorectal cancer screening program with FIT in Paris
- PMID: 29515157
- PMCID: PMC5841338
- DOI: 10.1038/s41598-018-22481-9
Results of the national organised colorectal cancer screening program with FIT in Paris
Abstract
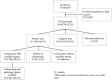
In France, colorectal cancer (CRC) benefits from a nationwide screening program. The faecal immunochemical test (FIT) is being used since April 2015. The test is recommended in asymptomatic patients followed by a colonoscopy if positive for identification and treatment of colorectal lesions. We investigate the CRC national organised screening program using FIT in Paris. We performed a retrospective observational study, collecting data from the screening program in Paris using the ADECA75 database. Rates of participation, numbers of positive FIT, detection rates and positive predictive values (PPV) for advanced adenomas (AA) and/or CRC were determined. Between 01/01/2016 and 30/06/2017, 620.227 Parisians were eligible and 409.340 were invited to participate to the program. A total of 88.796 participants (23%) performed the test with 3.839 positive tests (4.3%). In the positive test population, 2.706 out of 3.839 individuals (70.5%) performed the required colonoscopy with available reports. Histology reports were only available for 2.401 participants (88,7%). Regarding lesions, 733 (30,5%) and 205 patients (8.5%) had AA and CRC, respectively. Over 18 months of screening with FIT in Paris, the PPV is in line with expected results while the participation rate is below European recommendations.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous