Mechanisms of α-Synuclein Induced Synaptopathy in Parkinson's Disease
- PMID: 29515354
- PMCID: PMC5825910
- DOI: 10.3389/fnins.2018.00080
Mechanisms of α-Synuclein Induced Synaptopathy in Parkinson's Disease
Abstract
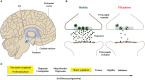
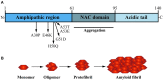
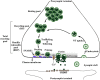
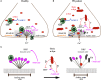
Parkinson's disease (PD) is characterized by intracellular inclusions of aggregated and misfolded α-Synuclein (α-Syn), and the loss of dopaminergic (DA) neurons in the brain. The resulting motor abnormalities mark the progression of PD, while non-motor symptoms can already be identified during early, prodromal stages of disease. Recent studies provide evidence that during this early prodromal phase, synaptic and axonal abnormalities occur before the degenerative loss of neuronal cell bodies. These early phenotypes can be attributed to synaptic accumulation of toxic α-Syn. Under physiological conditions, α-Syn functions in its native conformation as a soluble monomer. However, PD patient brains are characterized by intracellular inclusions of insoluble fibrils. Yet, oligomers and protofibrils of α-Syn have been identified to be the most toxic species, with their accumulation at presynaptic terminals affecting several steps of neurotransmitter release. First, high levels of α-Syn alter the size of synaptic vesicle pools and impair their trafficking. Second, α-Syn overexpression can either misregulate or redistribute proteins of the presynaptic SNARE complex. This leads to deficient tethering, docking, priming and fusion of synaptic vesicles at the active zone (AZ). Third, α-Syn inclusions are found within the presynaptic AZ, accompanied by a decrease in AZ protein levels. Furthermore, α-Syn overexpression reduces the endocytic retrieval of synaptic vesicle membranes during vesicle recycling. These presynaptic alterations mediated by accumulation of α-Syn, together impair neurotransmitter exocytosis and neuronal communication. Although α-Syn is expressed throughout the brain and enriched at presynaptic terminals, DA neurons are the most vulnerable in PD, likely because α-Syn directly regulates dopamine levels. Indeed, evidence suggests that α-Syn is a negative modulator of dopamine by inhibiting enzymes responsible for its synthesis. In addition, α-Syn is able to interact with and reduce the activity of VMAT2 and DAT. The resulting dysregulation of dopamine levels directly contributes to the formation of toxic α-Syn oligomers. Together these data suggest a vicious cycle of accumulating α-Syn and deregulated dopamine that triggers synaptic dysfunction and impaired neuronal communication, ultimately causing synaptopathy and progressive neurodegeneration in Parkinson's disease.
Keywords: Parkinson's disease; SNARE complex; active zone; dopamine; neurodegeneration; synapse; synaptopathy; α-synuclein.
Figures

References
-
- Alerte T. N. M., Akinfolarin A. A., Friedrich E. E., Mader S. A., Hong C.-S., Perez R. G. (2008). Alpha-synuclein aggregation alters tyrosine hydroxylase phosphorylation and immunoreactivity: lessons from viral transduction of knockout mice. Neurosci. Lett. 435, 24–29. 10.1016/j.neulet.2008.02.014 - DOI - PMC - PubMed
Publication types
Grants and funding
- HIRTH/OCT13/868-792/MNDA_/Motor Neurone Disease Association/United Kingdom
- G-0714/PUK_/Parkinson's UK/United Kingdom
- HIRTH/OCT16/890-792/MNDA_/Motor Neurone Disease Association/United Kingdom
- G0701498/MRC_/Medical Research Council/United Kingdom
- MR/L010666/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

