Steroid Transport, Local Synthesis, and Signaling within the Brain: Roles in Neurogenesis, Neuroprotection, and Sexual Behaviors
- PMID: 29515356
- PMCID: PMC5826223
- DOI: 10.3389/fnins.2018.00084
Steroid Transport, Local Synthesis, and Signaling within the Brain: Roles in Neurogenesis, Neuroprotection, and Sexual Behaviors
Abstract
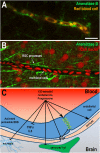
Sex steroid hormones are synthesized from cholesterol and exert pleiotropic effects notably in the central nervous system. Pioneering studies from Baulieu and colleagues have suggested that steroids are also locally-synthesized in the brain. Such steroids, called neurosteroids, can rapidly modulate neuronal excitability and functions, brain plasticity, and behavior. Accumulating data obtained on a wide variety of species demonstrate that neurosteroidogenesis is an evolutionary conserved feature across fish, birds, and mammals. In this review, we will first document neurosteroidogenesis and steroid signaling for estrogens, progestagens, and androgens in the brain of teleost fish, birds, and mammals. We will next consider the effects of sex steroids in homeostatic and regenerative neurogenesis, in neuroprotection, and in sexual behaviors. In a last part, we will discuss the transport of steroids and lipoproteins from the periphery within the brain (and vice-versa) and document their effects on the blood-brain barrier (BBB) permeability and on neuroprotection. We will emphasize the potential interaction between lipoproteins and sex steroids, addressing the beneficial effects of steroids and lipoproteins, particularly HDL-cholesterol, against the breakdown of the BBB reported to occur during brain ischemic stroke. We will consequently highlight the potential anti-inflammatory, anti-oxidant, and neuroprotective properties of sex steroid and lipoproteins, these latest improving cholesterol and steroid ester transport within the brain after insults.
Keywords: HDL; aromatase; blood-brain barrier; cholesterol; estrogens; lipoproteins; progestins; stroke.
Figures



References
-
- Alafuzoff I., Adolfsson R., Bucht G., Winblad B. (1983). Albumin and immunoglobulin in plasma and cerebrospinal fluid, and blood-cerebrospinal fluid barrier function in patients with dementia of Alzheimer type and multi-infarct dementia. J. Neurol. Sci. 60, 465–472. 10.1016/0022-510X(83)90157-0 - DOI - PubMed
-
- Alkayed N. J., Harukuni I., Kimes A. S., London E. D., Traystman R. J., Hurn P. D. (1998). Gender-linked brain injury in experimental stroke. Stroke 29, 159–165: discussion 166. - PubMed
-
- Alunni A., Vaccari S., Torcia S., Meomartini M. E., Nicotra A., Alfei L. (2005). Characterization of glial fibrillary acidic protein and astroglial architecture in the brain of a continuously growing fish, the rainbow trout. Eur. J. Histochem. 49, 157–166. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

