Junctional E-cadherin/p120-catenin Is Correlated with the Absence of Supporting Cells to Hair Cells Conversion in Postnatal Mice Cochleae
- PMID: 29515364
- PMCID: PMC5826362
- DOI: 10.3389/fnmol.2018.00020
Junctional E-cadherin/p120-catenin Is Correlated with the Absence of Supporting Cells to Hair Cells Conversion in Postnatal Mice Cochleae
Abstract
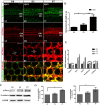
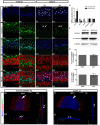
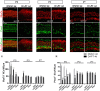
Notch inhibition is known to generate supernumerary hair cells (HCs) at the expense of supporting cells (SCs) in the mammalian inner ear. However, inhibition of Notch activity becomes progressively less effective at inducing SC-to-HC conversion in the postnatal cochlea and balance organs as the animal ages. It has been suggested that the SC-to-HC conversion capacity is inversely correlated with E-cadherin accumulation in postnatal mammalian utricles. However, whether E-cadherin localization is linked to the SC-to-HC conversion capacity in the mammalian inner ear is poorly understood. In the present study, we treated cochleae from postnatal day 0 (P0) with the Notch signaling inhibitor DAPT and observed apparent SC-to-HC conversion along with E-cadherin/p120ctn disruption in the sensory region. In addition, the SC-to-HC conversion capacity and E-cadherin/p120ctn disorganization were robust in the apex but decreased toward the base. We further demonstrated that the ability to regenerate HCs and the disruption of E-cadherin/p120ctn concomitantly decreased with age and ceased at P7, even after extended DAPT treatments. This timing is consistent with E-cadherin/p120ctn accumulation in the postnatal cochleae. These results suggest that the decreasing capacity of SCs to transdifferentiate into HCs correlates with E-cadherin/p120ctn localization in the postnatal cochleae, which might account for the absence of SC-to-HC conversion in the mammalian cochlea.
Keywords: DAPT; E-cadherin/p120ctn complexes; SC-to-HC conversion; cochlea; hair cells; supporting cells.
Figures




Similar articles
-
The postnatal accumulation of junctional E-cadherin is inversely correlated with the capacity for supporting cells to convert directly into sensory hair cells in mammalian balance organs.J Neurosci. 2011 Aug 17;31(33):11855-66. doi: 10.1523/JNEUROSCI.2525-11.2011. J Neurosci. 2011. PMID: 21849546 Free PMC article.
-
Spontaneous Hair Cell Regeneration Is Prevented by Increased Notch Signaling in Supporting Cells.Front Cell Neurosci. 2018 May 4;12:120. doi: 10.3389/fncel.2018.00120. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29780306 Free PMC article.
-
Wnt activation followed by Notch inhibition promotes mitotic hair cell regeneration in the postnatal mouse cochlea.Oncotarget. 2016 Oct 11;7(41):66754-66768. doi: 10.18632/oncotarget.11479. Oncotarget. 2016. PMID: 27564256 Free PMC article.
-
Cochlear hair cell regeneration after noise-induced hearing loss: Does regeneration follow development?Hear Res. 2017 Jun;349:182-196. doi: 10.1016/j.heares.2016.12.011. Epub 2016 Dec 26. Hear Res. 2017. PMID: 28034617 Free PMC article. Review.
-
Hair cell regeneration from inner ear progenitors in the mammalian cochlea.Am J Stem Cells. 2020 Jun 15;9(3):25-35. eCollection 2020. Am J Stem Cells. 2020. PMID: 32699655 Free PMC article. Review.
Cited by
-
YAP Mediates Hair Cell Regeneration in Balance Organs of Chickens, But LATS Kinases Suppress Its Activity in Mice.J Neurosci. 2020 May 13;40(20):3915-3932. doi: 10.1523/JNEUROSCI.0306-20.2020. Epub 2020 Apr 27. J Neurosci. 2020. PMID: 32341094 Free PMC article.
-
Vestibular Deficits in Deafness: Clinical Presentation, Animal Modeling, and Treatment Solutions.Front Neurol. 2022 Apr 4;13:816534. doi: 10.3389/fneur.2022.816534. eCollection 2022. Front Neurol. 2022. PMID: 35444606 Free PMC article. Review.
-
Localization of cadherins in the postnatal cochlear epithelium and their relation to space formation.Dev Dyn. 2024 Aug;253(8):771-780. doi: 10.1002/dvdy.692. Epub 2024 Jan 24. Dev Dyn. 2024. PMID: 38264972 Free PMC article.
-
Heme Oxygenase-1 Protects Hair Cells From Gentamicin-Induced Death.Front Cell Neurosci. 2022 Apr 13;16:783346. doi: 10.3389/fncel.2022.783346. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35496911 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

