Polymethoxyflavones from Nicotiana plumbaginifolia (Solanaceae) Exert Antinociceptive and Neuropharmacological Effects in Mice
- PMID: 29515437
- PMCID: PMC5826308
- DOI: 10.3389/fphar.2018.00085
Polymethoxyflavones from Nicotiana plumbaginifolia (Solanaceae) Exert Antinociceptive and Neuropharmacological Effects in Mice
Abstract
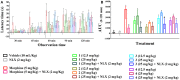
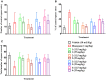
Polymethoxylavones (PMFs) are known to exhibit significant anti-inflammatory and neuroprotective properties. Nicotiana plumbaginifolia, an annual Bangladeshi herb, is rich in polymethoxyflavones that possess significant analgesic and anxiolytic activities. The present study aimed to determine the antinociceptive and neuropharmacological activities of polyoxygenated flavonoids namely- 3,3',5,6,7,8-hexamethoxy-4',5'-methylenedioxyflavone (1), 3,3',4',5',5,6,7,8-octamethoxyflavone (exoticin) (2), 6,7,4',5'-dimethylenedioxy-3,5,3'-trimethoxyflavone (3), and 3,3',4',5,5',8-hexamethoxy-6,7-methylenedioxyflavone (4), isolated and identified from N. plumbaginifolia. Antinociceptive activity was assessed using the acetic-acid induced writhing, hot plate, tail immersion, formalin and carrageenan-induced paw edema tests, whereas neuropharmacological effects were evaluated in the hole cross, open field and elevated plus maze test. Oral treatment of compounds 1, 3, and 4 (12.5-25 mg/kg b.w.) exhibited dose-dependent and significant (p < 0.01) antinociceptive activity in the acetic-acid, formalin, carrageenan, and thermal (hot plate)-induced pain models. The association of ATP-sensitive K+ channel and opioid systems in their antinociceptive effect was obvious from the antagonist effect of glibenclamide and naloxone, respectively. These findings suggested central and peripheral antinociceptive activities of the compounds. Compound 1, 3, and 4 (12.5 mg/kg b.w.) demonstrated significant (p < 0.05) anxiolytic-like activity in the elevated plus-maze test, while the involvement of GABAA receptor in the action of compound 3 and 4 was evident from the reversal effects of flumazenil. In addition, compounds 1 and 4 (12.5-25 mg/kg b.w) exhibited anxiolytic activity without altering the locomotor responses. The present study suggested that the polymethoxyflavones (1-4) from N. Plumbaginifolia could be considered as suitable candidates for the development of analgesic and anxiolytic agents.
Keywords: Nicotiana plumbaginifolia; antinociceptive; anxiolytic; benzodiazepine; opioid; polymethoxyflavone.
Figures




Similar articles
-
Antinociceptive and neuropharmacological activities of methanol extract of Phoenix sylvestris fruit pulp.Front Pharmacol. 2015 Oct 2;6:212. doi: 10.3389/fphar.2015.00212. eCollection 2015. Front Pharmacol. 2015. PMID: 26483687 Free PMC article.
-
Antinociceptive activities of Artocarpus lacucha Buch-ham (Moraceae) and its isolated phenolic compound, catechin, in mice.BMC Complement Altern Med. 2019 Aug 14;19(1):214. doi: 10.1186/s12906-019-2565-x. BMC Complement Altern Med. 2019. PMID: 31412852 Free PMC article.
-
Antinociceptive and anti-inflammatory effects of Memora nodosa and allantoin in mice.J Ethnopharmacol. 2016 Jun 20;186:298-304. doi: 10.1016/j.jep.2016.04.010. Epub 2016 Apr 11. J Ethnopharmacol. 2016. PMID: 27079223
-
Evaluation of anti-nociceptive and anti-inflammatory activities of the methanol extract of Holigarna caustica (Dennst.) Oken leaves.J Ethnopharmacol. 2019 May 23;236:401-411. doi: 10.1016/j.jep.2019.01.025. Epub 2019 Jan 28. J Ethnopharmacol. 2019. PMID: 30703495
-
Antinociceptive and anti-inflammatory properties of Tetracera alnifolia Willd. (Dilleniaceae) hydroethanolic leaf extract.J Basic Clin Physiol Pharmacol. 2018 Oct 17;30(2):173-184. doi: 10.1515/jbcpp-2016-0190. J Basic Clin Physiol Pharmacol. 2018. PMID: 30332392
Cited by
-
Potential inhibitors of SARS-CoV-2 (COVID 19) spike protein of the delta and delta plus variant: In silico studies of medicinal plants of North-East India.Curr Res Pharmacol Drug Discov. 2021;2:100065. doi: 10.1016/j.crphar.2021.100065. Epub 2021 Oct 22. Curr Res Pharmacol Drug Discov. 2021. PMID: 34870160 Free PMC article.
-
Phenolics as GABAA Receptor Ligands: An Updated Review.Molecules. 2022 Mar 8;27(6):1770. doi: 10.3390/molecules27061770. Molecules. 2022. PMID: 35335130 Free PMC article. Review.
-
The anti-nociceptive and anti-inflammatory effects of Pelargonium graveolens essential oil nanoemulsion in mice.Sci Rep. 2025 May 6;15(1):15754. doi: 10.1038/s41598-025-00722-y. Sci Rep. 2025. PMID: 40328821 Free PMC article.
-
Clerodendrum wallichii Merr Methanol Extract Protected Alcohol-Induced Liver Injury in Sprague-Dawley Rats by Modulating Antioxidant Enzymes.Evid Based Complement Alternat Med. 2022 Aug 23;2022:5635048. doi: 10.1155/2022/5635048. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36051496 Free PMC article.
-
5-HT1A Serotonergic, α-Adrenergic and Opioidergic Receptors Mediate the Analgesic Efficacy of Vortioxetine in Mice.Molecules. 2021 May 28;26(11):3242. doi: 10.3390/molecules26113242. Molecules. 2021. PMID: 34071269 Free PMC article.
References
-
- Aragão G. F., Carneiro L. M., Junior A. P., Vieira L. C., Bandeira P. N., Lemos T. L., et al. . (2006). A possible mechanism for anxiolytic and antidepressant effects of alpha- and beta-amyrin from Protium heptaphyllum (Aubl.) March. Pharmacol. Biochem. Behav. 85, 827–834. 10.1016/j.pbb.2006.11.019 - DOI - PubMed
-
- Bagdas D., Wilkerson J. L., Kulkarni A., Toma W., AlSharari S., Gul Z., et al. . (2016). The α7 nicotinic receptor dual allosteric agonist and positive allosteric modulator GAT107 reverses nociception in mouse models of inflammatory and neuropathic pain. Br. J. Pharmacol. 173, 2506–2520. 10.1111/bph.13528 - DOI - PMC - PubMed
-
- Bhargava H. N., Gulati A., Ramarao P. (1989). Effect of chronic administration of U-50,488H on tolerance to its pharmacological actions and on multiple opioid receptors in rat brain regions and spinal cord. J. Pharmacol. Exp. Ther. 251, 21–26. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

