GnRH-(1-5) Inhibits TGF-β Signaling to Regulate the Migration of Immortalized Gonadotropin-Releasing Hormone Neurons
- PMID: 29515521
- PMCID: PMC5826220
- DOI: 10.3389/fendo.2018.00045
GnRH-(1-5) Inhibits TGF-β Signaling to Regulate the Migration of Immortalized Gonadotropin-Releasing Hormone Neurons
Abstract
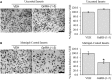
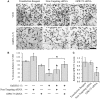
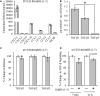
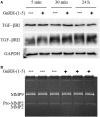
Gonadotropin-releasing hormone (GnRH) neurons originate outside the central nervous system (CNS) in the nasal placode where their migration to the basal forebrain is dependent on the integration of multiple signaling cues during development. The proper migration and establishment of the GnRH neuronal population within the CNS are critical for normal pubertal onset and reproductive function. The endopeptidase EP24.15 is expressed along the migratory path of GnRH neurons and cleaves the full-length GnRH to generate the metabolite GnRH-(1-5). Using the GN11 cell model, which is considered a pre-migratory GnRH neuronal cell line, we demonstrated that GnRH-(1-5) inhibits cellular migration in a wound closure assay by binding the orphan G protein-coupled receptor 173 (GPR173). In our current experiments, we sought to utilize an in vitro migration assay that better reflects the external environment that migrating GnRH neurons are exposed to during development. Therefore, we used a transwell assay where the inserts were coated with or without a matrigel, a gelatinous mixture containing extracellular matrix (ECM) proteins, to mimic the extracellular environment. Interestingly, GnRH-(1-5) inhibited the ability of GN11 cells to migrate only through ECM mimetic and was dependent on GPR173. Furthermore, we found that GN11 cells secrete TGF-β1, 2, and 3 but only TGF-β1 release and signaling were inhibited by GnRH-(1-5). To identify potential mechanisms involved in the proteolytic activation of TGF-β, we measured a panel of genes implicated in ECM remodeling. We found that GnRH-(1-5) consistently increased tissue inhibitors of metalloproteinase 1 expression, which is an inhibitor of proteinase activity, leading to a decrease in bioactive TGF-β and subsequent signaling. These results suggest that GnRH-(1-5) activating GPR173 may modulate the response of migrating GnRH neurons to external cues present in the ECM environment via an autocrine-dependent mechanism involving TGF-β.
Keywords: EP24.15; G protein-coupled receptor 173; G protein-coupled receptors; gonadotropin-releasing hormone; migration.
Figures
Similar articles
-
The metabolite GnRH-(1-5) inhibits the migration of immortalized GnRH neurons.Endocrinology. 2013 Feb;154(2):783-95. doi: 10.1210/en.2012-1746. Epub 2013 Jan 15. Endocrinology. 2013. PMID: 23321696
-
β-Arrestin 2 is a mediator of GnRH-(1-5) signaling in immortalized GnRH neurons.Endocrinology. 2013 Dec;154(12):4726-36. doi: 10.1210/en.2013-1286. Epub 2013 Oct 18. Endocrinology. 2013. PMID: 24140715
-
Hepatocyte growth factor acts as a motogen and guidance signal for gonadotropin hormone-releasing hormone-1 neuronal migration.J Neurosci. 2007 Jan 10;27(2):431-45. doi: 10.1523/JNEUROSCI.4979-06.2007. J Neurosci. 2007. PMID: 17215404 Free PMC article.
-
Gonadotropin-releasing hormone neuronal migration.Semin Reprod Med. 2007 Sep;25(5):305-12. doi: 10.1055/s-2007-984736. Semin Reprod Med. 2007. PMID: 17710726 Review.
-
Developmental aspect of the gonadotropin-releasing hormone system.Mol Cell Endocrinol. 2001 Dec 20;185(1-2):173-84. doi: 10.1016/s0303-7207(01)00616-5. Mol Cell Endocrinol. 2001. PMID: 11738807 Review.
Cited by
-
Comprehensive therapeutics targeting the corticospinal tract following spinal cord injury.J Zhejiang Univ Sci B. 2019 Mar.;20(3):205-218. doi: 10.1631/jzus.B1800280. J Zhejiang Univ Sci B. 2019. PMID: 30829009 Free PMC article. Review.
-
MicroRNA-124 Overexpression in Schwann Cells Promotes Schwann Cell-Astrocyte Integration and Inhibits Glial Scar Formation Ability.Front Cell Neurosci. 2020 Jul 2;14:144. doi: 10.3389/fncel.2020.00144. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32714149 Free PMC article.
-
Phoenixin-14 alters transcriptome and steroid profiles in female green-spotted puffer (Dichotomyctere nigroviridis).Sci Rep. 2022 Jun 8;12(1):9454. doi: 10.1038/s41598-022-13695-z. Sci Rep. 2022. PMID: 35676522 Free PMC article.
-
Proximity Interactome Analysis of Super Conserved Receptors Expressed in the Brain Identifies EPB41L2, SLC3A2, and LRBA as Main Partners.Cells. 2023 Nov 14;12(22):2625. doi: 10.3390/cells12222625. Cells. 2023. PMID: 37998360 Free PMC article.
-
Spatial and quantitative gene expression analysis of SREB receptors in the gonads of green-spotted pufferfish (Dichotomyctere nigroviridis).Gen Comp Endocrinol. 2025 Jan 1;360:114641. doi: 10.1016/j.ygcen.2024.114641. Epub 2024 Nov 12. Gen Comp Endocrinol. 2025. PMID: 39536984
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

