Novel Variant of New Delhi Metallo-β-lactamase, NDM-20, in Escherichia coli
- PMID: 29515538
- PMCID: PMC5826333
- DOI: 10.3389/fmicb.2018.00248
Novel Variant of New Delhi Metallo-β-lactamase, NDM-20, in Escherichia coli
Erratum in
-
Corrigendum: Novel Variant of New Delhi Metallo-β-lactamase, NDM-20, in Escherichia coli.Front Microbiol. 2018 Mar 23;9:497. doi: 10.3389/fmicb.2018.00497. eCollection 2018. Front Microbiol. 2018. PMID: 29611554 Free PMC article.
Abstract
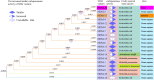
The spread of carbapenem-resistant Enterobacteriaceae (CRE) mediated by New Delhi metallo-β-lactamase (NDM) poses a serious challenge to clinicians and has become a major public health concern. NDM has been evolving into variants that possess different hydrolysis activity toward antibiotics, so as to affect treatment strategy. In addition, very few studies on NDM variants have focused on animal-derived bacterial isolates. Our study reports a novel NDM variant, NDM-20, in an isolate of Escherichia coli CCD1 recovered from the food animal swine in China. The isolate that was assigned to ST1114, exhibited high level resistance to all β-lactams tested, including aztreonam and carbapenems. The gene of blaNDM-20 was located on an IncX3-type plasmid, surrounded by multiple insertion sequences. Sequencing analysis demonstrated that blaNDM-20 contained three point mutations at positions 262 (G→T), 460 (A→C), and 809 (G→A), compared with blaNDM-1, and just one point mutation at position 809 (G→A), relative to blaNDM-5. Functional analysis revealed that the blaNDM-20 transformant, DH5α+pHSG398/NDM-20, exhibited a higher resistance to ertapenem than that of blaNDM-1 transformant DH5α+pHSG398/NDM-1. Kinetic parameter analysis showed that NDM-20 had increased enzymatic activity against some penicillins and cephalosporins but decreased carbapenemase activity relative to NDM-5. The identification of NDM-20 further confirms the evolution and prevalence of NDM variants in bacteria of food-animal origin.
Keywords: IncX3; NDM-20; ST1114; carbapenemase; kinetic parameters.
Figures


References
-
- Clinical and Laboratory Standards Institute [CLSI] (2015). Performance Standards for Antimicrobial Susceptibility Testing; 24th Informational Supplement, M100-S24. Wayne, PA: Clinical and Laboratory Standards Institute (CLSI).
-
- EUCAST (2017). European Committee on Antimicrobial Susceptibility Testing. http://www.eucast.org/clinical_breakpoints/
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

