Staphylococcus aureus Membrane-Derived Vesicles Promote Bacterial Virulence and Confer Protective Immunity in Murine Infection Models
- PMID: 29515544
- PMCID: PMC5826277
- DOI: 10.3389/fmicb.2018.00262
Staphylococcus aureus Membrane-Derived Vesicles Promote Bacterial Virulence and Confer Protective Immunity in Murine Infection Models
Abstract
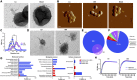
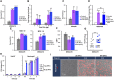
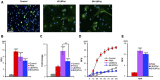
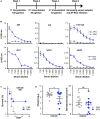
Staphylococcus aureus produces membrane-derived vesicles (MVs), which share functional properties to outer membrane vesicles. Atomic force microscopy revealed that S. aureus-derived MVs are associated with the bacterial surface or released into the surrounding environment depending on bacterial growth conditions. By using a comparative proteomic approach, a total of 131 and 617 proteins were identified in MVs isolated from S. aureus grown in Luria-Bertani and brain-heart infusion broth, respectively. Purified S. aureus MVs derived from the bacteria grown in either media induced comparable levels of cytotoxicity and neutrophil-activation. Administration of exogenous MVs increased the resistance of S. aureus to killing by whole blood or purified human neutrophils ex vivo and increased S. aureus survival in vivo. Finally, immunization of mice with S. aureus-derived MVs induced production of IgM, total IgG, IgG1, IgG2a, and IgG2b resulting in protection against subcutaneous and systemic S. aureus infection. Collectively, our results suggest S. aureus MVs can influence bacterial-host interactions during systemic infections and provide protective immunity in murine models of infection.
Keywords: Staphylococcus aureus; membrane-derived vesicles; protective immunity; proteomics; systemic infection.
Figures
Comment in
-
Commentary: Staphylococcus aureus Membrane-Derived Vesicles Promote Bacterial Virulence and Confer Protective Immunity in Murine Infection Models.Front Microbiol. 2018 Oct 1;9:2346. doi: 10.3389/fmicb.2018.02346. eCollection 2018. Front Microbiol. 2018. PMID: 30327647 Free PMC article. No abstract available.
Similar articles
-
Staphylococcus aureus produces membrane-derived vesicles that induce host cell death.PLoS One. 2011;6(11):e27958. doi: 10.1371/journal.pone.0027958. Epub 2011 Nov 16. PLoS One. 2011. PMID: 22114730 Free PMC article.
-
Bacterial membrane vesicles shape Staphylococcus aureus skin colonization and induction of innate immune responses.Exp Dermatol. 2022 Mar;31(3):349-361. doi: 10.1111/exd.14478. Epub 2021 Oct 29. Exp Dermatol. 2022. PMID: 34679243
-
Staphylococcus aureus Releases Proinflammatory Membrane Vesicles To Resist Antimicrobial Fatty Acids.mSphere. 2020 Sep 30;5(5):e00804-20. doi: 10.1128/mSphere.00804-20. mSphere. 2020. PMID: 32999082 Free PMC article.
-
The Role of Bacterial Membrane Vesicles in Human Health and Disease.Front Microbiol. 2022 Mar 1;13:828704. doi: 10.3389/fmicb.2022.828704. eCollection 2022. Front Microbiol. 2022. PMID: 35300484 Free PMC article. Review.
-
Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus.Front Cell Infect Microbiol. 2017 Jun 30;7:286. doi: 10.3389/fcimb.2017.00286. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28713774 Free PMC article. Review.
Cited by
-
Slightly acidic electrolyzed water inhibits inflammation induced by membrane vesicles of Staphylococcus aureus.Front Microbiol. 2024 Jan 12;14:1328055. doi: 10.3389/fmicb.2023.1328055. eCollection 2023. Front Microbiol. 2024. PMID: 38282743 Free PMC article.
-
Staphylococcus aureus membrane vesicles: an evolving story.Trends Microbiol. 2024 Nov;32(11):1096-1105. doi: 10.1016/j.tim.2024.04.003. Epub 2024 Apr 26. Trends Microbiol. 2024. PMID: 38677977 Review.
-
The Phospholipase A1 Activity of Glycerol Ester Hydrolase (Geh) Is Responsible for Extracellular 2-12(S)-Methyltetradecanoyl-Lysophosphatidylglycerol Production in Staphylococcus aureus.mSphere. 2023 Apr 20;8(2):e0003123. doi: 10.1128/msphere.00031-23. Epub 2023 Mar 28. mSphere. 2023. PMID: 36976028 Free PMC article.
-
Bacterial Membrane Vesicles: Physiological Roles, Infection Immunology, and Applications.Adv Sci (Weinh). 2023 Sep;10(25):e2301357. doi: 10.1002/advs.202301357. Epub 2023 Jun 25. Adv Sci (Weinh). 2023. PMID: 37357142 Free PMC article. Review.
-
Antibiotics Stimulate Formation of Vesicles in Staphylococcus aureus in both Phage-Dependent and -Independent Fashions and via Different Routes.Antimicrob Agents Chemother. 2019 Jan 29;63(2):e01439-18. doi: 10.1128/AAC.01439-18. Print 2019 Feb. Antimicrob Agents Chemother. 2019. PMID: 30509943 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

