Immunization Strategies Producing a Humoral IgG Immune Response against Devil Facial Tumor Disease in the Majority of Tasmanian Devils Destined for Wild Release
- PMID: 29515577
- PMCID: PMC5826075
- DOI: 10.3389/fimmu.2018.00259
Immunization Strategies Producing a Humoral IgG Immune Response against Devil Facial Tumor Disease in the Majority of Tasmanian Devils Destined for Wild Release
Abstract
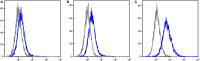
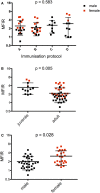
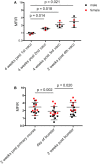
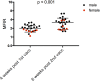
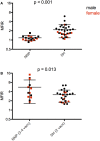
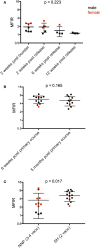
Devil facial tumor disease (DFTD) is renowned for its successful evasion of the host immune system. Down regulation of the major histocompatabilty complex class I molecule (MHC-I) on the DFTD cells is a primary mechanism of immune escape. Immunization trials on captive Tasmanian devils have previously demonstrated that an immune response against DFTD can be induced, and that immune-mediated tumor regression can occur. However, these trials were limited by their small sample sizes. Here, we describe the results of two DFTD immunization trials on cohorts of devils prior to their wild release as part of the Tasmanian Government's Wild Devil Recovery project. 95% of the devils developed anti-DFTD antibody responses. Given the relatively large sample sizes of the trials (N = 19 and N = 33), these responses are likely to reflect those of the general devil population. DFTD cells manipulated to express MHC-I were used as the antigenic basis of the immunizations in both trials. Although the adjuvant composition and number of immunizations differed between trials, similar anti-DFTD antibody levels were obtained. The first trial comprised DFTD cells and the adjuvant combination of ISCOMATRIX™, polyIC, and CpG with up to four immunizations given at monthly intervals. This compared to the second trial whereby two immunizations comprising DFTD cells and the adjuvant combination ISCOMATRIX™, polyICLC (Hiltonol®) and imiquimod were given a month apart, providing a shorter and, therefore, more practical protocol. Both trials incorporated a booster immunization given up to 5 months after the primary course. A key finding was that devils in the second trial responded more quickly and maintained their antibody levels for longer compared to devils in the first trial. The different adjuvant combination incorporating the RNAase resistant polyICLC and imiquimod used in the second trial is likely to be responsible. The seroconversion in the majority of devils in these anti-DFTD immunization trials was remarkable, especially as DFTD is hallmarked by its immune evasion mechanisms. Microsatellite analyzes of MHC revealed that some MHC-I microsatellites correlated to stronger immune responses. These trials signify the first step in the long-term objective of releasing devils with immunity to DFTD into the wild.
Keywords: Tasmanian devil facial tumour disease; adjuvant; humoral immunity/antibody response; vaccination; wild immunology.
Figures
Similar articles
-
The toll-like receptor ligands Hiltonol® (polyICLC) and imiquimod effectively activate antigen-specific immune responses in Tasmanian devils (Sarcophilus harrisii).Dev Comp Immunol. 2017 Nov;76:352-360. doi: 10.1016/j.dci.2017.07.004. Epub 2017 Jul 6. Dev Comp Immunol. 2017. PMID: 28689773
-
Evidence for induction of humoral and cytotoxic immune responses against devil facial tumor disease cells in Tasmanian devils (Sarcophilus harrisii) immunized with killed cell preparations.Vaccine. 2015 Jun 12;33(26):3016-25. doi: 10.1016/j.vaccine.2015.01.039. Epub 2015 Feb 20. Vaccine. 2015. PMID: 25708088
-
Regression of devil facial tumour disease following immunotherapy in immunised Tasmanian devils.Sci Rep. 2017 Mar 9;7:43827. doi: 10.1038/srep43827. Sci Rep. 2017. PMID: 28276463 Free PMC article.
-
Devil Facial Tumor Disease.Vet Pathol. 2016 Jul;53(4):726-36. doi: 10.1177/0300985815616444. Epub 2015 Dec 13. Vet Pathol. 2016. PMID: 26657222 Review.
-
Lessons learnt from the Tasmanian devil facial tumour regarding immune function in cancer.Mamm Genome. 2018 Dec;29(11-12):731-738. doi: 10.1007/s00335-018-9782-3. Epub 2018 Sep 17. Mamm Genome. 2018. PMID: 30225648 Review.
Cited by
-
Conserving adaptive potential: lessons from Tasmanian devils and their transmissible cancer.Conserv Genet. 2019 Feb;20(1):81-87. doi: 10.1007/s10592-019-01157-5. Epub 2019 Feb 14. Conserv Genet. 2019. PMID: 31551664 Free PMC article.
-
Changes in humoral immunity, myocardial damage, trace elements, and inflammatory factor levels in children with rotavirus enteritis.Am J Transl Res. 2022 Jan 15;14(1):452-459. eCollection 2022. Am J Transl Res. 2022. PMID: 35173864 Free PMC article.
-
No Evidence for Distinct Transcriptomic Subgroups of Devil Facial Tumor Disease (DFTD).Evol Appl. 2025 Apr 1;18(4):e70091. doi: 10.1111/eva.70091. eCollection 2025 Apr. Evol Appl. 2025. PMID: 40177324 Free PMC article.
-
Darwin, the devil, and the management of transmissible cancers.Conserv Biol. 2021 Apr;35(2):748-751. doi: 10.1111/cobi.13644. Epub 2020 Oct 8. Conserv Biol. 2021. PMID: 32992406 Free PMC article. No abstract available.
-
The immunopeptidomes of two transmissible cancers and their host have a common, dominant peptide motif.Immunology. 2021 Jun;163(2):169-184. doi: 10.1111/imm.13307. Epub 2021 Feb 4. Immunology. 2021. PMID: 33460454 Free PMC article.
References
-
- Hawkins CE, McCallum H, Mooney N, Jones M, Holdsworth M. Sarcophilus harrisii. In IUCN Red List of Threatened Species Version 2016-2. e.T40540A10331066 ed. International Union for the Conservation of Nature; (2008).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

