Endothelial to Mesenchymal Transition Represents a Key Link in the Interaction between Inflammation and Endothelial Dysfunction
- PMID: 29515588
- PMCID: PMC5826197
- DOI: 10.3389/fimmu.2018.00294
Endothelial to Mesenchymal Transition Represents a Key Link in the Interaction between Inflammation and Endothelial Dysfunction
Abstract
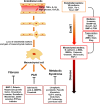
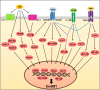
Endothelial cells that line the inner walls of blood vessels are in direct contact with blood and display remarkable heterogeneity in their response to exogenous stimuli. These ECs have unique location-dependent properties determined by the corresponding vascular beds and play an important role in regulating the homeostasis of the vascular system. Evidence suggests that vascular endothelial cells exposed to various environments undergo dynamic phenotypic switching, a key biological program in the context of endothelial heterogeneity, but that might result in EC dysfunction and, in turn, cause a variety of human diseases. Emerging studies show the importance of endothelial to mesenchymal transition (EndMT) in endothelial dysfunction during inflammation. EndMT is a complex biological process in which ECs lose their endothelial characteristics, acquire mesenchymal phenotypes, and express mesenchymal cell markers, such as alpha smooth muscle actin and fibroblast-specific protein 1. EndMT is induced by inflammatory responses, leading to pathological states, including tissue fibrosis, pulmonary arterial hypertension, and atherosclerosis, via dysfunction of the vascular system. Although the mechanisms associated with inflammation-induced EndMT have been identified, unraveling the specific role of this phenotypic switching in vascular dysfunction remains a challenge. Here, we review the current understanding on the interactions between inflammatory processes, EndMT, and endothelial dysfunction, with a focus on the mechanisms that regulate essential signaling pathways. Identification of such mechanisms will guide future research and could provide novel therapeutic targets for the treatment of vascular diseases.
Keywords: endothelial dysfunction; endothelial heterogeneity; endothelial to mesenchymal transition; inflammatory process; vascular disease.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

