Optical coherence tomography in neuromyelitis optica spectrum disorders: potential advantages for individualized monitoring of progression and therapy
- PMID: 29515685
- PMCID: PMC5833887
- DOI: 10.1007/s13167-017-0123-5
Optical coherence tomography in neuromyelitis optica spectrum disorders: potential advantages for individualized monitoring of progression and therapy
Abstract
Neuromyelitis optica spectrum disorders (NMOSD) are mostly relapsing inflammatory disorders of the central nervous system (CNS). Optic neuritis (ON) is the first NMOSD-related clinical event in 55% of the patients, which causes damage to the optic nerve and leads to visual impairment. Retinal optical coherence tomography (OCT) has emerged as a promising method for diagnosis of NMOSD and potential individual monitoring of disease course and severity. OCT not only detects damage to the afferent visual system caused by ON but potentially also NMOSD-specific intraretinal pathology, i.e. astrocytopathy. This article summarizes retinal involvement in NMOSD and reviews OCT methods that could be used now and in the future, for differential diagnosis, for monitoring of disease course, and in clinical trials.
Keywords: Diagnosis, differential; Disease progression; Neuromyelitis optica; Optic neuritis; Retina; Tomography, optical coherence; Vision disorders.
Conflict of interest statement
Compliance with ethical standardsF. C. Oertel reports no conflicts of interest. H. Zimmermann received speaker honorary from TEVA and Bayer Healthcare, independent from this work. A. U. Brandt received consulting fees unrelated to this study for research from Novartis, Biogen, Motognosis, Teva, and Bayer. FP received research support from the German Ministry for Education and Research (BMBF/KKNMS; Competence Network Multiple Sclerosis), the Deutsche Forschungsgemeinschaft (DFG) (grant exc. 257), and from the Guthy Jackson Charitable Foundation and National Multiple Sclerosis Society as well as research grants and speaker honoraria from Bayer, Teva, Genzyme, Merck, Novartis, MedImmune and is member of the steering committee of the OCTIMS study (Novartis).
Figures

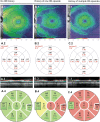

References
-
- Jarius S, Paul F, Franciotta D, Waters P, Zipp F, Hohlfeld R, et al. Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol. 2008;4(4):202–14. 10.1038/ncpneuro0764. - PubMed
-
- Kremer L, Mealy M, Jacob A, Nakashima I, Cabre P, Bigi S, et al. Brainstem manifestations in neuromyelitis optica: a multicenter study of 258 patients. Mult Scler. 2014;20(7):843–7. 10.1177/1352458513507822. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

