Identification of allosteric disulfides from labile bonds in X-ray structures
- PMID: 29515832
- PMCID: PMC5830721
- DOI: 10.1098/rsos.171058
Identification of allosteric disulfides from labile bonds in X-ray structures
Abstract
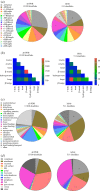
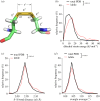
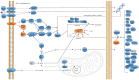
Protein disulfide bonds link pairs of cysteine sulfur atoms and are either structural or functional motifs. The allosteric disulfides control the function of the protein in which they reside when cleaved or formed. Here, we identify potential allosteric disulfides in all Protein Data Bank X-ray structures from bonds that are present in some molecules of a protein crystal but absent in others, or present in some structures of a protein but absent in others. We reasoned that the labile nature of these disulfides signifies a propensity for cleavage and so possible allosteric regulation of the protein in which the bond resides. A total of 511 labile disulfide bonds were identified. The labile disulfides are more stressed than the average bond, being characterized by high average torsional strain and stretching of the sulfur-sulfur bond and neighbouring bond angles. This pre-stress likely underpins their susceptibility to cleavage. The coagulation, complement and oxygen-sensing hypoxia inducible factor-1 pathways, which are known or have been suggested to be regulated by allosteric disulfides, are enriched in proteins containing labile disulfides. The identification of labile disulfide bonds will facilitate the study of this post-translational modification.
Keywords: allosteric disulfides; coagulation; complement; oxygen-sensing; redox.
Conflict of interest statement
We declare we have no competing interests.
Figures



References
-
- Cook KM, Hogg PJ. 2013. Post-translational control of protein function by disulfide bond cleavage. Antioxid. Redox Signal. 18, 1987–2015. (doi:10.1089/ars.2012.4807) - DOI - PubMed
-
- Butera D, Cook KM, Chiu J, Wong JW, Hogg PJ. 2014. Control of blood proteins by functional disulfide bonds. Blood 123, 2000–2007. (doi:10.1182/blood-2014-01-549816) - DOI - PMC - PubMed
-
- Hogg PJ. 2013. Targeting allosteric disulphide bonds in cancer. Nat. Rev. Cancer 13, 425–431. (doi:10.1038/nrc3519) - DOI - PubMed
-
- Wouters MA, Lau KK, Hogg PJ. 2004. Cross-strand disulphides in cell entry proteins: poised to act. Bioessays 26, 73–79. (doi:10.1002/bies.10413) - DOI - PubMed
-
- Bekendam RH, et al. 2016. A substrate-driven allosteric switch that enhances PDI catalytic activity. Nat. Commun. 7, 12579 (doi:10.1038/ncomms12579) - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
