Establishing and Maintaining an Extensive Library of Patient-Derived Xenograft Models
- PMID: 29515970
- PMCID: PMC5825907
- DOI: 10.3389/fonc.2018.00019
Establishing and Maintaining an Extensive Library of Patient-Derived Xenograft Models
Abstract
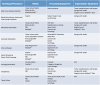
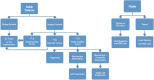
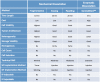
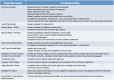
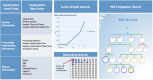
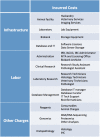
Patient-derived xenograft (PDX) models have recently emerged as a highly desirable platform in oncology and are expected to substantially broaden the way in vivo studies are designed and executed and to reshape drug discovery programs. However, acquisition of patient-derived samples, and propagation, annotation and distribution of PDXs are complex processes that require a high degree of coordination among clinic, surgery and laboratory personnel, and are fraught with challenges that are administrative, procedural and technical. Here, we examine in detail the major aspects of this complex process and relate our experience in establishing a PDX Core Laboratory within a large academic institution.
Keywords: patient sample acquisition; patient-derived xenograft; patient-derived xenograft database; patient-derived xenograft implantation techniques; patient-derived xenograft propagation techniques.
Figures





References
-
- Mattar M, Abdel-Wahab O, de Stanchina E. Chapter 2 – Acquisition and storage of clinical samples to establish PDX models. In: Uthamanthyl R, Tinkey P, editors. Patient Derived Tumor Xenograft Models. London: Academic Press; (2017). p. 109–18.
-
- Mattar M, Abdel-Wahab O, Poirier JT, Scaltriti M, de Stanchina E. Chapter 3 – Methodologies for developing and maintaining patient-derived xenograft mouse models. In: Uthamanthyl R, Tinkey P, editors. Patient Derived Tumor Xenograft Models. London: Academic Press; (2017). p. 119–34.
-
- Thompson-Iritani S, Schmechel SC. Chapter 1 – Regulations of patient-derived xenografts. In: Uthamanthyl R, Tinkey P, editors. Patient Derived Tumor Xenograft Models. London: Academic Press; (2017). p. 93–108.
-
- Summary of the HIPAA Privacy Rule. (2016). Available from: https://www.hhs.gov/hipaa/for-professionals/privacy/laws-regulations/ind...
-
- Krivtsov A, Mattar M, Uthamanthil RK, de Stanchina E. Chapter 6 – Running a PDX core laboratory or a PDX support program. In: Uthamanthyl R, Tinkey P, editors. Patient Derived Tumor Xenograft Models. London: Academic Press; (2017). p. 161–72.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

