Exosome-Based Cell-Cell Communication in the Tumor Microenvironment
- PMID: 29515996
- PMCID: PMC5826063
- DOI: 10.3389/fcell.2018.00018
Exosome-Based Cell-Cell Communication in the Tumor Microenvironment
Abstract
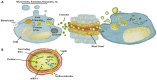
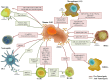
Tumors are not isolated entities, but complex systemic networks involving cell-cell communication between transformed and non-transformed cells. The milieu created by tumor-associated cells may either support or halt tumor progression. In addition to cell-cell contact, cells communicate through secreted factors via a highly complex system involving characteristics such as ligand concentration, receptor expression and integration of diverse signaling pathways. Of these, extracellular vesicles, such as exosomes, are emerging as novel cell-cell communication mediators in physiological and pathological scenarios. Exosomes, membrane vesicles of endocytic origin released by all cells (both healthy and diseased), ranging in size from 30 to 150 nm, transport all the main biomolecules, including lipids, proteins, DNAs, messenger RNAs and microRNA, and perform intercellular transfer of components, locally and systemically. By acting not only in tumor cells, but also in tumor-associated cells such as fibroblasts, endothelium, leukocytes and progenitor cells, tumor- and non-tumor cells-derived exosomes have emerged as new players in tumor growth and invasion, tumor-associated angiogenesis, tissue inflammation and immunologic remodeling. In addition, due to their property of carrying molecules from their cell of origin to the peripheral circulation, exosomes have been increasingly studied as sources of tumor biomarkers in liquid biopsies. Here we review the current literature on the participation of exosomes in the communication between tumor and tumor-associated cells, highlighting the role of this process in the setup of tumor microenvironments that modulate tumor initiation and metastasis.
Keywords: cancer; cell-cell communication; exosomes; extracellular vesicles (EVs); tumor microenvironment.
Figures
References
-
- Abdouh M., Hamam D., Gao Z. H., Arena V., Arena M., Arena G. O. (2017). Exosomes isolated from cancer patients' sera transfer malignant traits and confer the same phenotype of primary tumors to oncosuppressor-mutated cells. J. Exp. Clin. Cancer Res. 36, 113. 10.1186/s13046-017-0587-0 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

