Neural crest stem cells protect spinal cord neurons from excitotoxic damage and inhibit glial activation by secretion of brain-derived neurotrophic factor
- PMID: 29516218
- PMCID: PMC5949140
- DOI: 10.1007/s00441-018-2808-z
Neural crest stem cells protect spinal cord neurons from excitotoxic damage and inhibit glial activation by secretion of brain-derived neurotrophic factor
Abstract
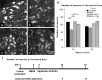
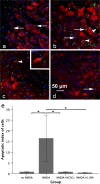
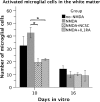
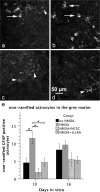
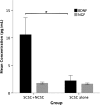
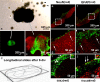
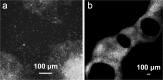
The acute phase of spinal cord injury is characterized by excitotoxic and inflammatory events that mediate extensive neuronal loss in the gray matter. Neural crest stem cells (NCSCs) can exert neuroprotective and anti-inflammatory effects that may be mediated by soluble factors. We therefore hypothesize that transplantation of NCSCs to acutely injured spinal cord slice cultures (SCSCs) can prevent neuronal loss after excitotoxic injury. NCSCs were applied onto SCSCs previously subjected to N-methyl-D-aspartate (NMDA)-induced injury. Immunohistochemistry and TUNEL staining were used to quantitatively study cell populations and apoptosis. Concentrations of neurotrophic factors were measured by ELISA. Migration and differentiation properties of NCSCs on SCSCs, laminin, or hyaluronic acid hydrogel were separately studied. NCSCs counteracted the loss of NeuN-positive neurons that was otherwise observed after NMDA-induced excitotoxicity, partly by inhibiting neuronal apoptosis. They also reduced activation of both microglial cells and astrocytes. The concentration of brain-derived neurotrophic factor (BDNF) was increased in supernatants from SCSCs cultured with NCSCs compared to SCSCs alone and BDNF alone mimicked the effects of NCSC application on SCSCs. NCSCs migrated superficially across the surface of SCSCs and showed no signs of neuronal or glial differentiation but preserved their expression of SOX2 and Krox20. In conclusion, NCSCs exert neuroprotective, anti-apoptotic and glia-inhibitory effects on excitotoxically injured spinal cord tissue, some of these effects mediated by secretion of BDNF. However, the investigated NCSCs seem not to undergo neuronal or glial differentiation in the short term since markers indicative of an undifferentiated state were expressed during the entire observation period.
Keywords: Apoptosis; Excitotoxicity; Neuroprotection; Secretion of soluble factors; Suppressed glial activation.
Figures
Similar articles
-
Differential Neuroprotective Effects of Interleukin-1 Receptor Antagonist on Spinal Cord Neurons after Excitotoxic Injury.Neuroimmunomodulation. 2017;24(4-5):220-230. doi: 10.1159/000484607. Epub 2018 Jan 26. Neuroimmunomodulation. 2017. PMID: 29393213
-
Epidermal neural crest stem cell-derived glia enhance neurotrophic elements in an ex vivo model of spinal cord injury.J Cell Biochem. 2018 Apr;119(4):3486-3496. doi: 10.1002/jcb.26520. Epub 2018 Jan 11. J Cell Biochem. 2018. PMID: 29143997
-
Synergic effects of EPI-NCSCs and OECs on the donor cells migration, the expression of neurotrophic factors, and locomotor recovery of contused spinal cord of rats.J Mol Neurosci. 2015 Mar;55(3):760-9. doi: 10.1007/s12031-014-0416-2. Epub 2014 Sep 20. J Mol Neurosci. 2015. PMID: 25239519
-
Epidermal neural crest stem cells and their use in mouse models of spinal cord injury.Brain Res Bull. 2010 Oct 30;83(5):189-93. doi: 10.1016/j.brainresbull.2010.07.002. Epub 2010 Jul 14. Brain Res Bull. 2010. PMID: 20637266 Review.
-
Neural Crest-Derived Stem Cells (NCSCs) Obtained from Dental-Related Stem Cells (DRSCs): A Literature Review on Current Knowledge and Directions toward Translational Applications.Int J Mol Sci. 2022 Feb 28;23(5):2714. doi: 10.3390/ijms23052714. Int J Mol Sci. 2022. PMID: 35269856 Free PMC article. Review.
Cited by
-
Empty mesoporous silica particles significantly delay disease progression and extend survival in a mouse model of ALS.Sci Rep. 2020 Nov 26;10(1):20675. doi: 10.1038/s41598-020-77578-x. Sci Rep. 2020. PMID: 33244084 Free PMC article.
-
Human Embryonic Stem Cell-derived Neural Crest Cells Promote Sprouting and Motor Recovery Following Spinal Cord Injury in Adult Rats.Cell Transplant. 2021 Jan-Dec;30:963689720988245. doi: 10.1177/0963689720988245. Cell Transplant. 2021. PMID: 33522309 Free PMC article.
-
Therapeutic potential of NGF-enriched extracellular vesicles in modulating neuroinflammation and enhancing peripheral nerve remyelination.Acta Neuropathol Commun. 2025 May 27;13(1):118. doi: 10.1186/s40478-025-02033-9. Acta Neuropathol Commun. 2025. PMID: 40420167 Free PMC article.
-
Cardiac Neural Crest and Cardiac Regeneration.Cells. 2022 Dec 28;12(1):111. doi: 10.3390/cells12010111. Cells. 2022. PMID: 36611905 Free PMC article. Review.
-
Neural crest-like stem cells for tissue regeneration.Stem Cells Transl Med. 2021 May;10(5):681-693. doi: 10.1002/sctm.20-0361. Epub 2021 Feb 2. Stem Cells Transl Med. 2021. PMID: 33533168 Free PMC article. Review.
References
-
- Aldskogius H, Berens C, Kanaykina N, Liakhovitskaia A, Medvinsky A, Sandelin M, Schreiner S, Wegner M, Hjerling-Leffler J, Kozlova EN. Regulation of boundary cap neural crest stem cell differentiation after transplantation. Stem Cells. 2009;27(7):1592–1603. doi: 10.1002/stem.77. - DOI - PMC - PubMed
-
- Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7(10):R100. doi: 10.1186/gb-2006-7-10-r100. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

