Development of a polarized pancreatic ductular cell epithelium for physiological studies
- PMID: 29517421
- PMCID: PMC6086968
- DOI: 10.1152/japplphysiol.00043.2018
Development of a polarized pancreatic ductular cell epithelium for physiological studies
Abstract
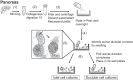
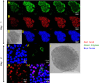
Pancreatic ductular epithelial cells comprise the majority of duct cells in pancreas, control cystic fibrosis transmembrane conductance regulator (CFTR)-dependent bicarbonate ([Formula: see text]) secretion, but are difficult to grow as a polarized monolayer. Using NIH-3T3-J2 fibroblast feeder cells and a Rho-associated kinase inhibitor, we produced well-differentiated and polarized porcine pancreatic ductular epithelial cells. Cells grown on semipermeable filters at the air-liquid interface developed typical epithelial cell morphology and stable transepithelial resistance and expressed epithelial cell markers (zona occludens-1 and β-catenin), duct cell markers (SOX-9 and CFTR), but no acinar (amylase) or islet cell (chromogranin) markers. Polarized cells were studied in Ussing chambers bathed in Krebs-Ringer [Formula: see text] solution at 37°C gassed with 5% CO2 to measure short-circuit currents ( Isc). Ratiometric measurement of extracellular pH was performed with fluorescent SNARF-conjugated dextran at 5% CO2. Cells demonstrated a baseline Isc (12.2 ± 3.2 μA/cm2) that increased significantly in response to apical forskolin-IBMX (∆ Isc: 35.4 ± 3.8 μA/cm2, P < 0.001) or basolateral secretin (∆ Isc: 31.4 ± 2.5 μA/cm2, P < 0.001), both of which increase cellular levels of cAMP. Subsequent addition of apical GlyH-101, a CFTR inhibitor, decreased the current (∆ Isc: 20.4 ± 3.8 μA/cm2, P < 0.01). Extracellular pH and [Formula: see text] concentration increased significantly after forskolin-IBMX (pH: 7.18 ± 0.23 vs. 7.53 ± 0.19; [Formula: see text] concentration, 14.5 ± 5.9 vs. 31.8 ± 13.4 mM; P < 0.05 for both). We demonstrate the development of a polarized pancreatic ductular epithelial cell epithelium with CFTR-dependent [Formula: see text] secretion in response to secretin and cAMP. This model is highly relevant, as porcine pancreas physiology is very similar to humans and pancreatic damage in the cystic fibrosis pig model recapitulates that of humans. NEW & NOTEWORTHY Pancreas ductular epithelial cells control cystic fibrosis transmembrane conductance regulator (CFTR)-dependent bicarbonate secretion. Their function is critical because when CFTR is deficient in cystic fibrosis bicarbonate secretion is lost and the pancreas is damaged. Mechanisms that control pancreatic bicarbonate secretion are incompletely understood. We generated well-differentiated and polarized porcine pancreatic ductular epithelial cells and demonstrated feasibility of bicarbonate secretion. This novel method will advance our understanding of pancreas physiology and mechanisms of bicarbonate secretion.
Keywords: bicarbonate secretion; cystic fibrosis; duct cells; pancreas.
Figures





Similar articles
-
Physiology and pathophysiology of bicarbonate secretion by pancreatic duct epithelium.Nagoya J Med Sci. 2012 Feb;74(1-2):1-18. Nagoya J Med Sci. 2012. PMID: 22515107 Free PMC article. Review.
-
Transepithelial bicarbonate secretion: lessons from the pancreas.Cold Spring Harb Perspect Med. 2012 Oct 1;2(10):a009571. doi: 10.1101/cshperspect.a009571. Cold Spring Harb Perspect Med. 2012. PMID: 23028131 Free PMC article.
-
Cystic fibrosis transmembrane conductance regulator currents in guinea pig pancreatic duct cells: inhibition by bicarbonate ions.Gastroenterology. 2000 Jun;118(6):1187-96. doi: 10.1016/s0016-5085(00)70372-6. Gastroenterology. 2000. PMID: 10833494
-
Guanylin in the human pancreas: a novel luminocrine regulatory pathway of electrolyte secretion via cGMP and CFTR in the ductal system.Histochem Cell Biol. 2001 Feb;115(2):131-45. doi: 10.1007/s004180000244. Histochem Cell Biol. 2001. PMID: 11444148
-
The Physiology and Pathophysiology of Pancreatic Ductal Secretion: The Background for Clinicians.Pancreas. 2015 Nov;44(8):1211-33. doi: 10.1097/MPA.0000000000000421. Pancreas. 2015. PMID: 26465950 Review.
Cited by
-
Pancreatic duct organoid swelling is chloride-dependent.J Cyst Fibros. 2024 Jan;23(1):169-171. doi: 10.1016/j.jcf.2023.08.003. Epub 2023 Aug 24. J Cyst Fibros. 2024. PMID: 37633792 Free PMC article.
-
Pro-inflammatory cytokines stimulate CFTR-dependent anion secretion in pancreatic ductal epithelium.Cell Mol Biol Lett. 2024 Jan 23;29(1):18. doi: 10.1186/s11658-024-00537-1. Cell Mol Biol Lett. 2024. PMID: 38262945 Free PMC article.
-
In utero and postnatal VX-770 administration rescues multiorgan disease in a ferret model of cystic fibrosis.Sci Transl Med. 2019 Mar 27;11(485):eaau7531. doi: 10.1126/scitranslmed.aau7531. Sci Transl Med. 2019. PMID: 30918114 Free PMC article.
-
Mouse pancreatic ductal organoid culture as a relevant model to study exocrine pancreatic ion secretion.Lab Invest. 2020 Jan;100(1):84-97. doi: 10.1038/s41374-019-0300-3. Epub 2019 Aug 13. Lab Invest. 2020. PMID: 31409889
-
Genome Editing in Ferret Airway Epithelia Mediated by CRISPR/Nucleases Delivered with Amphiphilic Shuttle Peptides.Hum Gene Ther. 2023 Aug;34(15-16):705-718. doi: 10.1089/hum.2023.016. Epub 2023 Jul 20. Hum Gene Ther. 2023. PMID: 37335046 Free PMC article.
References
-
- Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: clinical and pathological study. Am J Dis Child 56: 344–399, 1938. doi:10.1001/archpedi.1938.01980140114013. - DOI
-
- Borowitz D, Durie PR, Clarke LL, Werlin SL, Taylor CJ, Semler J, De Lisle RC, Lewindon P, Lichtman SM, Sinaasappel M, Baker RD, Baker SS, Verkade HJ, Lowe ME, Stallings VA, Janghorbani M, Butler R, Heubi J. Gastrointestinal outcomes and confounders in cystic fibrosis. J Pediatr Gastroenterol Nutr 41: 273–285, 2005. doi:10.1097/01.mpg.0000178439.64675.8d. - DOI - PubMed
-
- Brooks AM, Grossman MI. Postprandial pH and neutralizing capacity of the proximal duodenum in dogs. Gastroenterology 59: 85–89, 1970. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

