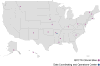
The institutional development award states pediatric clinical trials network: building research capacity among the rural and medically underserved
- PMID: 29517535
- PMCID: PMC5927618
- DOI: 10.1097/MOP.0000000000000597
The institutional development award states pediatric clinical trials network: building research capacity among the rural and medically underserved
Abstract
Purpose of review: The institutional development award (IDeA) program was created to increase the competitiveness of investigators in states with historically low success rates for National Institutes of Health (NIH) research funding applications. IDeA states have high numbers of rural and medically underserved residents with disproportionately high rates of infant mortality, obesity, and poverty. This program supports the development and expansion of research infrastructure and research activities in these states. The IDeA States Pediatric Clinical Trials Network (ISPCTN) is part of the environmental influences on child health outcomes program. Its purpose is to build research capacity within IDeA states and provide opportunities for children in IDeA states to participate in clinical trials. This review describes the current and future activities of the network.
Recent findings: In its initial year, the ISPCTN created an online series on clinical trials, initiated participation in a study conducted by the pediatric trials network, and proposed two novel clinical trials for obese children. Capacity building and clinical trial implementation will continue in future years.
Summary: The ISPCTN is uniquely poised to establish and support new pediatric clinical research programs in underserved populations, producing both short and long-term gains in the understanding of child health.
Conflict of interest statement
None
Figures
Similar articles
-
IDeA States Pediatric Clinical Trials Network for Underserved and Rural Communities.Pediatrics. 2020 Oct;146(4):e20200290. doi: 10.1542/peds.2020-0290. Epub 2020 Sep 17. Pediatrics. 2020. PMID: 32943534 Free PMC article.
-
Capacity Building for a New Multicenter Network Within the ECHO IDeA States Pediatric Clinical Trials Network.Front Pediatr. 2021 Jul 14;9:679516. doi: 10.3389/fped.2021.679516. eCollection 2021. Front Pediatr. 2021. PMID: 34336738 Free PMC article.
-
Team Science Process Builds Research Coordinators' Voices in a National Pediatric Clinical Trials Network.SOCRA Source. 2020 Aug;2020(105):68-73. SOCRA Source. 2020. PMID: 34354547 Free PMC article.
-
Coordination of the Environmental influences on Child Health Outcomes program: so the whole is greater than the sum of its parts.Curr Opin Pediatr. 2018 Apr;30(2):263-268. doi: 10.1097/MOP.0000000000000598. Curr Opin Pediatr. 2018. PMID: 29351109 Free PMC article. Review.
-
Demonstrating the value of orthopaedic surgery through multicenter trials: AOA critical issues.J Bone Joint Surg Am. 2015 Apr 1;97(7):e35. doi: 10.2106/JBJS.N.00159. J Bone Joint Surg Am. 2015. PMID: 25834087 Review.
Cited by
-
The ACT NOW Clinical Practice Survey: Gaps in the Care of Infants With Neonatal Opioid Withdrawal Syndrome.Hosp Pediatr. 2019 Aug;9(8):585-592. doi: 10.1542/hpeds.2019-0089. Epub 2019 Jul 19. Hosp Pediatr. 2019. PMID: 31324654 Free PMC article.
-
Protocol for the Vitamin D Oral Replacement in Asthma (VDORA) study.Contemp Clin Trials. 2022 Sep;120:106861. doi: 10.1016/j.cct.2022.106861. Epub 2022 Jul 28. Contemp Clin Trials. 2022. PMID: 35907490 Free PMC article.
-
Geographic inequality in funding by National Institutes of Health negatively impacts almost one-half of the states in the United States.Front Public Health. 2024 Sep 25;12:1452494. doi: 10.3389/fpubh.2024.1452494. eCollection 2024. Front Public Health. 2024. PMID: 39386949 Free PMC article. Review.
-
Rationale and protocol for a cluster randomized, cross-over trial of recruitment methods of rural children in primary care clinics: A feasibility study of a pediatric weight control trial in the IDeA States Pediatric Clinical Trials Network.Contemp Clin Trials. 2021 Aug;107:106476. doi: 10.1016/j.cct.2021.106476. Epub 2021 Jun 9. Contemp Clin Trials. 2021. PMID: 34118426 Free PMC article.
-
IDeA States Pediatric Clinical Trials Network for Underserved and Rural Communities.Pediatrics. 2020 Oct;146(4):e20200290. doi: 10.1542/peds.2020-0290. Epub 2020 Sep 17. Pediatrics. 2020. PMID: 32943534 Free PMC article.
References
-
- Bolen S, Tilburt J, Baffi C, et al. Defining "success" in recruitment of underrepresented populations to cancer clinical trials: moving toward a more consistent approach. Cancer. 2006;106(6):1197–204. - PubMed
-
- Slora EJ, Harris DL, Bocian AB, Wasserman RC. Pediatric clinical research networks: current status, common challenges, and potential solutions. Pediatrics. 2010;126(4):740–5. - PubMed
-
- Schilsky RL, McIntyre OR, Holland JF, Frei E., 3rd A concise history of the cancer and leukemia group B. Clin Cancer Res. 2006;12(11 Pt 2):3553s–5s. - PubMed
Publication types
MeSH terms
Grants and funding
- UG1 OD024945/OD/NIH HHS/United States
- UG1 HD090903/HD/NICHD NIH HHS/United States
- UG1 OD030016/OD/NIH HHS/United States
- UG1 OD024943/OD/NIH HHS/United States
- UG1 OD024947/OD/NIH HHS/United States
- UG1 OD024958/OD/NIH HHS/United States
- UG1 OD024942/OD/NIH HHS/United States
- UG1 HD090875/HD/NICHD NIH HHS/United States
- UG1 OD024944/OD/NIH HHS/United States
- UG1 OD024946/OD/NIH HHS/United States
- UG1 HD090849/HD/NICHD NIH HHS/United States
- UG1 OD024955/OD/NIH HHS/United States
- UG1 OD024953/OD/NIH HHS/United States
- UG1 OD024950/OD/NIH HHS/United States
- UG1 OD024952/OD/NIH HHS/United States
- UG1 OD024951/OD/NIH HHS/United States
- UG1 OD024948/OD/NIH HHS/United States
- UG1 OD024959/OD/NIH HHS/United States
- U24 OD024957/OD/NIH HHS/United States
- UG1 OD024954/OD/NIH HHS/United States
- UG1 OD024956/OD/NIH HHS/United States
- UG1 OD024949/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials