The efficiency of endothelin receptor antagonist bosentan for pulmonary arterial hypertension associated with congenital heart disease: A systematic review and meta-analysis
- PMID: 29517668
- PMCID: PMC5882424
- DOI: 10.1097/MD.0000000000010075
The efficiency of endothelin receptor antagonist bosentan for pulmonary arterial hypertension associated with congenital heart disease: A systematic review and meta-analysis
Abstract
Background: Oral bosentan has been widely applied in pulmonary arterial hypertension associated with congenital heart disease (PAH-CHD). A systemic review and meta-analysis was conducted for a therapeutic evaluation of oral bosentan in both adult and pediatric patients with PAH-CHD. The acute responses and a long-term effect were respectively assessed in a comparison with baseline characteristics, and the improvement of exercise tolerance was analyzed.
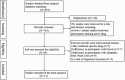
Methods: PubMed, Medline, Embase, and Cochrane Central Register of clinical controlled trails or observational studies have been searched for a recording of bosentan effects on the PAH-CHD participants. For mortality and rate of adverse events (AEs), it was described in detail. Randomized-effects model or fixed-effects model was used to calculate different effective values with a sensitivity analysis.
Results: Seventeen studies were pooled in this review, and 3 studies enrolled the pediatric patients. Among all studies, 456 patients were diagnosed with PAH-CHD, and 91.7% were treated with oral bosentan. With a term less than 6 months of bosentan therapy, there existed a significant improvement in 6-minute walk distance (6MWD) and the World Health Organization functional class (WHO-FC), but no such differences in Borg dyspnea index scores (BDIs) and the resting oxygen saturation (SpO2). Although with a prolonged treatment, not only 6MWD and FC, but also the resting SpO2 and heart rate were changed for a better exercise capability. Additionally, compared with the basic cardiopulmonary hemodynamics, it showed a statistically significant difference in mean pulmonary arterial pressure (mPAP) and pulmonary vascular resistance index (PVRi). Although a limitation of pooled studies with comparative outcomes of different terms, outcomes presented a lower WHO-FC which contributes to a success in a prolonged treatment.
Conclusions: Bosentan in PAH-CHD is well established and still requires clinical trials for an identification of its efficiency on CHD patients for an optimized period lessening a serious complication and the common AEs.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- Mclaughlin VV, Davis M, Cornwell W. Pulmonary arterial hypertension. Curr Probl Cardiol 2011;36:461–508. - PubMed
-
- Liu HL, Chen XY, Li JR, et al. Efficacy and safety of PAH-specific therapy in pulmonary arterial hypertension: a meta-analysis of randomized clinical trials. Chest 2016;150:353–66. - PubMed
-
- Zuckerman WA, Krishnan U, Rosenzweig EB. Pulmonary arterial hypertension associated with congenital heart disease. Curr Pediatrics Rep 2012;21:328–37. - PubMed
-
- Beghetti M, Tissot C. Pulmonary arterial hypertension in congenital heart diseases [C]. Seminars in respiratory and critical care medicine. Semin Respir Crit Care Med 2009;421–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical